Section 11
Handling and Analysis of Samples
Interim Final – June, 2024
Section 11.0 Handling and Analysis of Samples – Interim Final – November 12, 2008
Section 11.1 Sample Containers
11.1.1 Soil Sample Containers
11.1.1.1 Non-Volatile Soil Sample Containers
11.1.1.2 Volatile Soil Sample Containers
11.1.1.3 Multi-Increment Soil Sample Containers
11.1.2 Water Sample Containers
11.1.2.1 Non-Volatile Water Sample Containers
11.1.2.2 Volatile Water Sample Containers
Section 11.2 Sample Preservation and Maximum Hold Times
11.2.1 Sample Temperature
11.2.1.1 Bulk Soil Samples To Be Processed Using Multi Increment Sampling
11.2.2 Chemical Preservation
11.2.3 Sample Maximum Hold Times
Section 11.3 Sample Control and Chain-of-Custody Procedures
11.3.1 Sample Identification and Labels
11.3.2 Chain-of-Custody
11.3.2.1 Chain-of-Custody Forms
11.3.2.2 Chain-of-Custody Seals
Section 11.4 Sample Shipping
11.4.1 Sample Packing
11.4.2 Shipping or Delivery
Section 11.5 Approved Analytical Methods
Section 11.6 Field-Based Measurement Technologies
Figures
11-1 Example Chain-of-Custody Form
Appendices
11-A Laboratory Methods, Containers, Preservation, and Holding Times for Soil Samples
11-B Laboratory Methods, Containers, Preservation, and Holding Times for Groundwater Samples
Return to the Top of the Page
11.0 HANDLING AND ANALYSIS OF SAMPLES
This section presents appropriate methods for containing, preserving, shipping, and analyzing environmental samples for projects conducted under the guidance of this Technical Guidance Manual (TGM). Proper handling and analysis of environmental samples is an essential step to collecting representative, defensible data and to achieving Data Quality Objectives (DQO). The Sampling and Analysis Plan (SAP) documents necessary sample handling and analysis procedures and should identify the matrix to be sampled, the type and volume of proposed sample containers, preservatives (if any), and the proposed analytical methods for each sampling event.
This section focuses on soil and groundwater samples collected as part of an environmental investigation, which are sent to a fixed laboratory for analysis. Mobile laboratories used for field analysis may also be employed during an environmental investigation, but the use of mobile laboratories should be carefully planned and documented in the SAP prior to field activities and analysis. The State of Hawaiʻi Department of Health (HDOH) Hazard Evaluation and Emergency Response Office (HEER Office) recommends the submittal of SAPs proposing the use of mobile laboratories for review prior to the initiation of sample collection activities.
During the collection, handling, and packing activities, personnel should use proper personal protective equipment (PPE). Typical PPE for use in sample collection, handling, and packing should include gloves and safety glasses, especially when employing chemical preservatives. Site-specific conditions may require the use of additional PPE (e.g., chemical protection clothing) based on the hazards present at the site, which should be documented in the site-specific Safety and Health Plan within the SAP.
Packing extra sample containers is recommended when planning fieldwork, in case additional sampling locations or areas are identified during execution of the fieldwork. Breakage, both prior to and after sample collection should be considered, particularly in light of challenges presented by remote field sites, as well as shipping requirements unique to performing investigations in Hawaiʻi.
Guidance is presented on the use of soil sampling equipment in Section 5 and on the use of groundwater or surface water sampling equipment in Section 6. A discussion of gas phase samples is not included in this section; guidance on handling and analysis of soil vapor or indoor air samples is presented in Section 7. Quality assurance/quality control (QA/QC) considerations, such as trip blanks and equipment rinsate blanks, are discussed in Section 10.
11.1 SAMPLE CONTAINERS
Recommended sample containers for soil are presented in the table in Appendix 11-A, while the table in the Appendix 11-B presents the recommended sample containers for groundwater. The sample volumes for the containers listed in the tables represent the recommended size of container. More than one sample container may be required for the associated parameter and method. If soil or groundwater samples are to be analyzed for multiple contaminants, the sample volumes listed in the tables will require adjustment. Always consult with the laboratory when planning fieldwork to ensure that the proper sample containers and preservatives are used and sufficient sample mass/volume is collected for all intended analyses.
11.1.1 SOIL SAMPLE CONTAINERS
The types of sample containers used for the collection of soil samples is dependent upon the characteristics of media to be sampled as well as the specific analysis to be performed. Other factors, such as the anticipated concentrations of contaminants and the desired reporting limits are also important to consider when selecting appropriate soil sample containers.
In general, soil sample containers may be grouped into the following three broad categories: (1) non-volatile soil sample containers, (2) volatile soil sample containers, and (3) Multi-Increment soil sample containers (note that Multi-Increment samples may include both non-volatile and volatile analysis).
11.1.1.1 NON-VOLATILE SOIL SAMPLE CONTAINERS
Soil samples for non-volatile analysis are typically collected in wide-mouth glass jars sealed with Teflon-lined caps. Various volumes of wide-mouth glass jars, ranging from 2 ounces to 16 ounces, are employed in soil sample collection and are available as certified pre-cleaned prior to shipment to the end user. During sample collection, the soil is transferred directly from the sampling device (e.g., split-spoon sampler or acrylic tube liner) to the glass jar, which is sealed with a Teflon-lined cap.
Soil samples for non-volatile analysis may also be collected in stainless steel or brass tubes sealed with Teflon tape and plastic end caps, or in some cases in plastic bags. The stainless steel or brass tubes are typically 6 inches in length and vary between 1.5 and 3 inches or greater in diameter. The tubes are typically placed as a liner in a split spoon sampler driven by a drill rig. During sample collection, the soil is retained in the tubes following retrieval from the sampler, and Teflon tape and a plastic end cap is immediately placed over each end of the tube.
11.1.1.2 VOLATILE SOIL SAMPLE CONTAINERS
United States Environmental Protection Agency (USEPA) Method 5035 describes a closed-system purge-and-trap process for the analysis of volatile organic compounds in solid materials (e.g., soils, sediments, and solid waste) (USEPA, 1997g), which was subsequently updated with Method 5035A (USEPA, 2002h). The method is designed for use on samples containing low levels of VOCs, but procedures are also provided for collecting and preparing solid samples containing high concentrations of VOCs and for oily wastes. The procedures in EPA Method 5035 and 5035A may be used in conjunction with any appropriate determinative gas chromatographic procedure, including, but not limited to, EPA Methods 8015, 8021, and 8260.
Soil samples for volatile analysis can be collected using an EnCore® sampler, TerraCore® sampler, or similar sampling device capable of collecting a known mass of soil, approximately 5 grams, for preservation and analysis. The soil aliquot in the sampler may be chemically preserved by placing it into a glass jar containing a known quantity of preservative (see Subsection 11.2.3 for additional discussion of volatile soil sample collection).
The use of glass jars without the use of chemical preservatives when collecting soil samples for volatile analysis is not recommended.
The use of stainless steel or brass tubes when collecting soil samples for volatile analysis is not recommended.
11.1.1.3 MULTI-INCREMENT SOIL SAMPLE CONTAINERS
The collection of a 30- to 50-increment sample can result in approximately 500 to 2,000 grams of soil (i.e., the Multi-Increment sample) depending upon the mass of each increment. The sample container for a Multi-Increment sample must be large enough to accommodate this mass. For non-volatile analysis, a common method for collecting a Multi-Increment bulk sample is to place each increment into a dedicated, disposal plastic bag (such as a 2 gallon zip top or a heavy duty trash compactor bag) as each increment is collected. The bag may be sealed, labeled and submitted directly to the laboratory for sub-sampling and analysis. There is a potential for some semi-volatile organic compounds (SVOCs), in particular, phthalates, to be transferred from plastic bags to samples, particularly if the soil has coarse or sharp particles that may abrade the bag surface. Therefore, when collecting Multi-Increment samples, the sampling team should consider the use of clean wide-mouth glass jars to collect and/or transport samples to be analyzed for SVOCs. These types of jars are available commercially and from Hawaiʻi-based analytical laboratories.
As discussed in Subsection 4.2.1, the Multi-Increment sample for non-volatile analysis may be sub-sampled in the field if an appropriate Standard Operating Procedure (SOP) has been developed for field sub-sampling and included in the SAP. In this case, the Multi-Increment sample would be collected in a dedicated, disposable plastic bag or wide-mouth jar, which is transferred to a pan for sieving and sub-sampling. The sub-sample is collected into a plastic zip top bag or glass jar and submitted to the laboratory for analysis. Field sub-sampling should follow procedures similar to those employed by the analytical laboratory as described in Subsection 4.2.2. Field sub-sampling of the Multi-Increment sample to collect a representative sub-sample may be difficult to perform due to conditions commonly encountered at field sites in Hawaiʻi (e.g., trade winds and/or rain). Laboratory sub-sampling to collect a representative sub-sample is generally preferable due to the controlled environmental conditions. Whenever samples are sub-sampled in the field, fully document the procedures, equipment, training requirements, and QA/QC measures employed during field sub-sampling in the SAP.
For laboratory sub-sampling, the most common QA/QC measure includes the collection of sub-sampling replicates (typically triplicates) to assess the error introduced by the sub-sampling routine. See discussion of replicates for laboratory sub-sampling in Subsection 10.7.6.
The recommended approach for the collection of Multi-Increment soil samples intended for volatile analysis is discussed in Subsection 4.2.7.
11.1.2 WATER SAMPLE CONTAINERS
The type of sample container used for collecting surface water or groundwater samples is dependent upon the specific analysis to be performed. Other factors, such as the anticipated concentrations of contaminants, the desired reporting limits, and the presence of free product are also important to consider when selecting appropriate water sample containers.
In general, water sample containers may be grouped into the following two broad categories: (1) non-volatile water sample containers, and (2) volatile water sample containers.
11.1.2.1 NON-VOLATILE WATER SAMPLE CONTAINERS
Water samples for organic non-volatile analysis are typically collected in 1 liter amber glass jars without the use of chemical preservatives. When collecting groundwater samples, fill the water sample containers by directing the outlet of the sampling device (i.e., pump tubing or bailer) toward the top and side of the sample container to allow the water to run down the inside of the bottle. Avoid agitation and the creation of bubbles when collecting water samples. To prevent cross contamination, avoid contacting the interior or top of the sample containers with either the sampling device or gloved hands.
Water samples for dissolved metals analysis are collected in 250 milliliter (mL) plastic bottles and preserved with nitric acid to a pH less than 2. Dissolved metals analysis also requires filtration prior to collection as discussed in Section 6.
11.1.2.2 VOLATILE WATER SAMPLE CONTAINERS
Water samples for volatile analysis are typically collected in 40 mL glass jars with septum-sealed screw caps and preserved with hydrochloric acid (HCl) to a pH less than 2. During sample collection, the screw caps should be carefully placed on the jars and sealed with zero headspace. When collecting groundwater samples for volatile analysis, fill the 40 mL jars by directing the outlet of the sampling device toward the top and side of the sample container to allow the water to run down the inside of the bottle. Adjust the flow rate of the water sampling device so that it does not cause the jars to rapidly overflow, causing loss of VOCs, sample volume, or any sample preservative. Avoid agitation and the creation of bubbles when collecting volatile water samples to prevent the loss of volatile constituents. To prevent cross contamination, avoid contacting the interior or top of the sample containers with either the sampling device or gloved hands.
The preservative HCl may react (effervesce) with turbid water containing calcareous particulates, resulting in a loss of volatile constituents. If a strong reaction is observed when filling the 40 mL jars containing HCl, collect the water samples for volatile analysis as unpreserved samples and note on the chain-of-custody. The collection of unpreserved water samples reduces the hold time of 14 days down to 7 days.
11.2 SAMPLE PRESERVATION AND HOLD TIMES
This section presents recommendations provided by the USEPA’s compendium of testing methods guidance SW846 for the recommended sample preservation for soil and water sample collection. Always consult with the laboratory when planning fieldwork to ensure that the proper sample containers and preservatives are used. Tables 11-A and 11-B, provided in the Appendices, present the recommended preservation and Maximum Hold Times (MHT) for soil and groundwater, respectively. MHTs and preservation requirements are provided to minimize significant change to contaminant concentrations between sampling and extraction because of intrinsic biogeochemical reactions, including biotic and abiotic degradation, volatilization, oxidation/reduction, or irreversible sorption. SW846 section four focuses on practices relevant to maintaining sample integrity and representativeness. The section is applicable to trace analyses. Some considerations may be less relevant for source level samples.
Sample preservation consists of methods to assure the samples analyzed in the laboratory are representative of the field conditions. Preservation methods may include maintaining sample temperatures, analyzing the samples within recommended MHTs or using chemicals (such as HCl or nitric acid [HNO3]) to stabilize the target contaminants by altering the sample chemistry.
11.2.1 SAMPLE TEMPERATURE
Upon collection of sample containers, immediately begin the cooling process in the field by placing the sample containers in an insulated cooler containing water ice or frozen gel packs. Use of water ice is generally considered by the HEER Office to be more efficient to rapidly cool samples, and may be especially important for use with samples for volatile analyses, when feasible. Maintain the temperature of the sample containers at less than or equal to 6 degrees Celsius (°C) from the time of collection through the delivery to the analytical laboratory.
Samples which require thermal preservation shall be considered acceptable if the arrival temperature is within 2°C of the required temperature or the method specified range. For samples with a temperature requirement of 4°C, an arrival temperature from 0°C and 6°C meets specifications. Samples that are delivered to the laboratory on the same day that they are collected may not meet these criteria. In these cases, the samples are considered acceptable if there is evidence that the chilling process has begun, such as arrival at the analytical laboratory on ice.
Freezing samples immediately following sample collection can extend the holding times significantly. Polynuclear Aromatic Hydrocarbons (PAHs) retained their integrity for 138 days or about ten times the expected MHT when frozen (EPA, 2005). The authors of method 1633 for the analysis of PFAS in various matrices put significant effort into evaluating meaningful MHTs. They found the MHT for water stored at 6ºC to be 28 days whereas freezing samples to -20ºC extended the MHT to 90 days.
11.2.1.1 BULK SOIL SAMPLES TO BE PROCESSED USING MULTI INCREMENT SAMPLING
According to the SW846 Soil samples are to be collected and placed on ice in the field to reduce the degradation of sample chemistry following sample collection. However, if samples are to be collected for bulk soil sample processing and will not be analyzed for volatile or semi volatile components, samples may be shipped without ice. For the purposes of this guidance, a chemical is considered to be semi-volatile if its vapor pressure is between 0.1- and 1.0-mm Hg or if it is a liquid at 25°C or if the Henry’s Law Constant exceeds 0.00001atm-m3/mol (USEPA, 2019b). Chemicals that fall into this category include Total Petroleum Hydrocarbon as diesel (TPHd), some PAHs and elemental mercury. A chemical is considered unstable if its half-life is less than 30 days (see Section 4, Appendix K, section k.6).
11.2.2 CHEMICAL PRESERVATION
Request sample containers with pre-measured chemical reagents from the analytical laboratory. If laboratory provided containers are not available, add the chemical reagents to the sample containers prior to mobilizing to the field. Carefully place the soil or groundwater samples into the sample containers to minimize loss of chemical preservative as well as volatile constituents in the sample (i.e., do not overfill water sample containers or leave the cap off a jar containing methanol for soil samples).
Some commonly used preservation chemicals may react with the sample media. For example, calcareous soil may react with sodium bisulfate; turbid groundwater collected from a coral aquifer formation may react with hydrochloric acid. If the sample media reacts with the chemical preservative, volatile organic constituents may be lost due to effervescence during sample collection, so an alternative preservation method or no chemical preservation may be needed. These considerations should be taken into account while preparing a Sampling and Analysis Plan (SAP) (see Section 3).
The use of chemical preservatives for fieldwork in Hawai`i presents challenges when shipping preserved samples between islands or to the mainland (see Subsection 11.4 for additional details on shipping preservatives).
Some samples collected for specific analysis, such as dissolved metals in groundwater, may require pre-treatment prior to collection, as well as preservation (see Section 6.0).
11.2.3 SAMPLE MAXIMUM HOLD TIMES
MHTs are established to ensure analytical methods are performed prior to the degradation of chemicals which may occur following the collection of samples for environmental analysis. However, some of the MHTs presented by the EPA are inaccurate and overly conservative. This is especially true for some recalcitrant semi volatile compounds with MHTs designated by the SW846 to be 7 to 14 days. United States Contract Laboratories and researchers within the EPA have been working to change the use of MHTs from absolute sample acceptance or rejection to sample advisory and narration. Issues occur when regulatory review of analyses trigger data to be flagged or rejected, often requiring expensive resampling and analysis. Tables 11-A and 11-B have been updated to include MHTs that have resulted from the USEPAs Sample Holding Time Reevaluation (USEPA, 2005). A well written SAP should consider potential impacts to MHTs and discuss how to use impacted data. Data Quality Objectives (DQO)s, such as obtaining representative samples may take precedence over the MHT given a particular analysis.
The following excerpt is provided by the USEPA in Section 4.0 of the SW846 version VII. “The preservation and holding time information presented in Table 4-1 does not represent EPA requirements, but rather is intended solely as guidance. Selection of preservation techniques and applicable holding times should be based on all available information, including the properties of the analytes of interest for the project, their anticipated concentration levels, the composition of the sample matrix itself, and the stated project-specific DQOs. A longer holding time may be appropriate if it can be demonstrated that the analytes of interest are not adversely affected from preservation, storage and analyses performed outside the recommended holding times.” For example, the collection of soil samples from a former sugar cane plantation for the evaluation of potential impacts from legacy organochlorine pesticides should be processed using a Multi Increment® Sampling approach. It is possible that the time required to collect all the field samples and process them would reach the current MHT for the analytical method. Understanding the recalcitrant nature of the pesticides being evaluated, their MHT should be given less concern compared to obtaining representative data. Surface soil samples collected from legacy sites in Hawaiʻi are less prone to degradation once samples are collected in laboratory jars and chilled than when the soil remained in-situ. The MHT of 14 days on the surface soil samples that are further preserved than their original pre-sampled state should be weighed considerably less than other DQOs.
Several methods provide guidelines for both the hold time until extraction (denoted by “E” in the tables in Appendix 11-A and Appendix 11-B) and the hold time after extraction until analysis (denoted by an “A” in the tables).
The MHT for several analyses have been increased to match recommendations from the EPA holding time reevaluation report. For example, the holding time for PCBs in water has been increased from 7 to 365 days. Results from samples analyzed past the hold times may be usable, depending upon the DQO set forth in the SAP. At a minimum, the results from analyses conducted past the recommended hold times should be interpreted as having a potential bias. The laboratory reporting the samples should provide narration describing the potential impact to the data.
Volatile Soil Samples
The use of Multi Increment sampling methods are recommended for testing of soil for VOCs (refer to Subsection 4.2.8.1). The use of discrete soil samples is discouraged, due to the small mass of soil represented by the laboratory data (e.g., five-grams). Sample containers, preservation, holding times and laboratory method for testing of VOCs in soil and water samples are described in EPA Method 5035 and 5035A and summarized in Appendices 11-A and 11-B, respectively (USEPA 1997g, 2002h).
The sample collection and preparation methods in EPA Method 5035 and 5035A include short hold times, ranging from 48 hours up to 14 days. The guidance recommends a number of potential methods including 1) preservation of soil samples in methanol, 2) freezing samples at low temperatures, 3) holding unpreserved samples for short time periods on ice, 4) preservation of soil with an acidic solution (sodium bisulfite), and 5) preservation of increments in reagent water. Preservation of calcareous, coralline soils with acidic solutions can cause the material to effervesce, resulting in a loss of VOCs. This could pose a problem in low lying coastal areas underlain by “caprock”, marine sediment, as well as fill material derived from these areas. The use of reagent-grade water has also come under question and is not recommended for use in Hawaiʻi, due to concern about extraction efficiency as well as the short hold time of 48 hours (refer to Section 5). Preservation of MI samples with methanol is preferred and considered most reliable, although freezing of increments (e.g. using dry ice or water ice with salt) and shipment to the laboratory for extraction in methanol is unavoidable in some cases. This is especially true for projects on islands other than Oʻahu, due to logistical and safety issues associated with the air transportation and storage of methanol.
MI soil sample preservation methods and associated hold times for VOC testing are presented below (in order of preference) and summarized in Appendix 11-A and Subsection 4.2.8.1:
- Preserved soil samples collected with a sampling device capable of providing a pre-determined mass of soil and immediately extruded into a glass jar containing methanol should be analyzed within 14 days from the time of sample collection. Studies have demonstrated that the integrity of several target VOCs is extended much longer than 14 days once preserved in methanol. Longer hold times are acceptable if required and should be discussed in SAP and with the HDOH prior to the start of work. This approach is recommended by the HEER Office when feasible in the field. Reporting limits achieved by the use of methanol might be elevated due to the need to dilute the solution for testing. If this is the case, the reporting limits can be used for general screening purposes in place of the applicable Environmental Action Levels (EALs; refer to HDOH, 2016). In this application, methanol acts as the preservative as well as the extraction solvent and a limited volume of methanol extract is introduced directly into the laboratory instrument. As such, the dilution and the reporting limits are higher than with some other preservation and extraction approaches. In some cases, the laboratory may be able achieve lower reporting limits for specific contaminants preserved in methanol by using additional analytical techniques, so consultation with the laboratory is recommended. The methanol preservation method may not be feasible due to the requirements or restrictions on hazardous material air shipments by the Department of Transportation (DOT) and/or the International Air Transportation Association (IATA). Consequently, applicable shipping regulations should be understood and carefully followed.
- Unpreserved soil increments collected in sealable, airtight sampling devices and immediately frozen in the field to less than negative 7°C should be analyzed within 14 days from the time of sample collection. Longer hold times are acceptable if required and should be discussed in the SAP and with the HDOH prior to the start of work. Note that sealable, airtight coring tools or vials should not be frozen to less than -20°C to prevent problems with the integrity of the seals. Dry ice in direct contact with the sample containers may freeze them below -20°C, so dry ice needs to be used appropriately to achieve the desired temperature range (for example use a layer of insulating material between the dry ice and sample containers). Alternately, bags of water ice mixed with table salt may be used to freeze samples to below -7°C in the field.
- Unpreserved soil increments collected with a sealable, airtight coring device (e.g., EnCore® samplers or equivalent device) or in sealable, airtight vials and stored at 4°C must be extracted and analyzed within 48 hours from the time of sample collection. However, analysis time may be extended to 14 days of the sample collection date if the unpreserved soil increments are either placed in methanol or frozen to <-7°C by the lab within 48 hours from the time of sample collection in field.
- Preserved soil samples collected with a sampling device capable of providing a pre-determined mass of soil and immediately extruded into a glass jar containing sodium bisulfate preservative in reagent water must be analyzed within 14 days from the time of sample collection. This approach is appropriate for non-calcareous soils. A field check to determine whether the soil is calcareous is recommended (calcareous formations are commonly encountered in coastal areas when performing fieldwork in Hawaiʻi). In addition, certain VOCs such as styrene, TCE, trichlorofluoromethane, cis- and trans 1,3-dichloropropene, 2-chloroethylvinyl ether, and vinyl chloride may be decomposed by the bisulfate leading to results biased low, so acid preservation should not be used for these contaminants of concern.
11.3 SAMPLE CONTROL AND CHAIN-OF-CUSTODY PROCEDURES
Sample control and chain-of-custody procedures are extremely important for establishing that sample integrity was maintained from the time of collection through the time of analysis. Sample control procedures include the use of unique sample identifications, and sample labeling requirements. Chain-of-custody procedures include the use of chain-of-custody forms and custody seals.
11.3.1 SAMPLE IDENTIFICATION AND LABELS
Each sample collected in the field must be provided with a unique sample identification. In general, sample identification may include some or all of the following information:
- Project number (if samples collected by a consultant)
- Project location
- Sample location information (i.e., borehole or monitoring well identification)
- Depth of sample collection (for subsurface soil samples)
- Date/Time reference
A date/time reference is recommended if multiple samples are anticipated to be collected over the course of the project (such as during long-term monitoring projects).
Each sample collected in the field must be properly labeled using laboratory supplied (or equivalent) labels completed with indelible ink. The sample labels should contain the following information:
- Sample identification number
- Sampling date
- Sampling time
- Type of preservation
- Analysis requested
- Initials of sampler
Additional sample information should be documented in the field log including, but not limited to, the following:
- sample collection method (manual sampling, direct push drill rig)
- type of sample (e.g. Multi-Increment or discrete)
- significant observations noted during sample collection (petroleum odor or staining in soil, petroleum product or sheen on water surface)
- a cross reference of primary and replicate QA/QC samples
The peel and stick sample label should be securely affixed to the sample container. The outside of the sample container should be thoroughly cleaned and dried prior to affixing the label. Application of clear plastic adhesive tape over the label on the sample container provides a secondary means of securing the label to the container. Use of an indelible pen to mark the sample identification number on the container is a good backup method that can be used to identify a sample container in the event that it gets separated from the label.
Do not identify or cross-reference QA/QC samples on the sample labels or on the chain-of-custody form (see Section 10 for additional guidance on QA/QC samples).
11.3.2 CHAIN-OF-CUSTODY
Chain-of-custody is the process by which authorized custody of a sample is successively transferred from one person to another by the use of approved procedures and documents. If sample integrity is to be defensible, chain-of-custody procedures are necessary to document handling of samples from procurement through final analysis and disposal.
A sample is considered to be under a person’s custody if:
- The sample is in the person’s physical possession.
- The sample is in view of the person after that person has taken possession.
- The sample is secured by that person so that no one can tamper with the sample.
- The sample is secured by that person in an area where access is restricted to authorized personnel.
11.3.2.1 CHAIN-OF-CUSTODY FORMS
Chain-of-custody forms are used for tracking the samples from the time of collection in the field through the time of analysis. The chain-of-custody form contains the following information:
- Project identification
- Sampler’s name
- Sender – Company name and address
- Destination – Laboratory name and address
- Sample identification
- Number of sample containers per sample
- Preservation, if any
- Date and time of sample collection for each sample
- Requested analyses
- Special handling requirements, if any
- Shipping company
- Printed name and signature of person relinquishing custody, and date and time when custody relinquished
- Printed name and signature of person receiving custody, and date and time when custody received.
Complete chain-of-custody forms at the time of sample collection and prior to leaving the field site. Analytical laboratories typically provide company-specific chain-of-custody forms, and sample labels, if sample containers are procured through the analytical laboratory. Complete chain-of-custody forms with indelible ink.
When transferring samples, the individuals involved must sign, date, and record the time in the relinquished/received-section on the form. The sampler retains one copy of the chain-of-custody form when relinquishing the samples following sample collection. Completely fill out all applicable sections of the form and numerically sequence the forms (i.e., page 1 of 3, etc.) if more than one chain-of-custody form is used for a sample batch. Consider grouping similar sample media in chain-of-custody batches for submittal to the laboratory (i.e., submit groundwater samples on one chain-of-custody batch separate from soil samples collected for the same project).
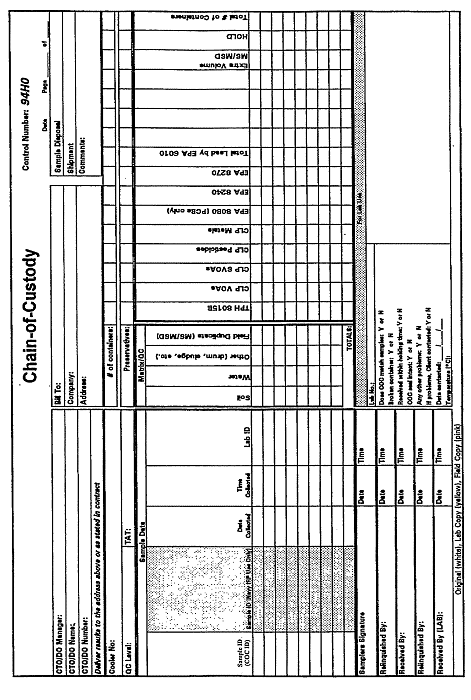
Figure 11-1. Example Chain-of-Custody Form.
[Source: US Navy, 2007]
Shipping companies (e.g. Federal Express, DHL, etc.) are not expected to sign the chain-of-custody form. However, complete shipping documentation, including tracking numbers, becomes part of the chain-of-custody record. When using shipping companies, the last person to have custody of the samples must fill in both the relinquished section (as normal) as well as the received section (identifying the shipping company’s name and the date and time when the shipment was given into custody of the shipping company).
Once received at the laboratory, custody procedures shall apply. It is then the laboratory’s responsibility to maintain custody records throughout sample preparation and analysis. An example generic chain-of-custody form is presented in Figure 11-1.
11.3.2.2 CHAIN-OF-CUSTODY SEALS
To ensure sample integrity when shipping samples, use custody seals. The custody seals must be dated and initialed by the personnel responsible for custody of the samples. Intact custody seals when the samples are logged into the laboratory indicate the physical integrity of the sample was not compromised during sample shipment.
Use custody seals on the outside of each container or cooler when shipping samples. Two seals are required, one at the front or opening edge of the cooler and one at the rear or hinged edge of the cooler. Clear tape can be applied over the custody seals when sealing the cooler or container for shipment.
Shipping sample coolers from the outer islands to Oʻahu or to mainland laboratories may require inspection by the Transportation Security Administration (TSA), which may break the chain-of-custody if custody seals are only used on the outside of shipping coolers. In this event, ensure individual sample integrity through the use of custody seals on individual sample container lids.
Custody seals may not be necessary if the sampling personnel retain custody of the samples from the time of collection to the time of delivery to the analytical lab, if the samples are delivered in person and a shipping company is not used.
11.4 SAMPLE SHIPPING
Upon completion of sample collection, shipment of samples to an analytical laboratory is typically required. This may include transport from an outer island to an Oʻahu-based laboratory or transport to a mainland laboratory. Properly pack the shipping containers to protect the individual sample containers, maintain the samples at temperature, and comply with all applicable transportation regulations.
Chain-of-custody forms are typically placed inside a sealed bag and adhered to the interior of the shipping container. The shipping paperwork is adhered to the outside of the shipping container and the container sealed for shipment. Shipping paperwork need only be attached to one cooler if multiple coolers are used in a single shipment, provided the shipping company concurs with this practice. When shipping multiple coolers in a single shipment, use additional labels to identify the cooler along with the total number of coolers in the shipment (e.g., cooler 2 of 3).
11.4.1 SAMPLE PACKING
Standard coolers are typically employed when packing samples for shipment although other shipping containers are acceptable. Interior packing materials include bubble wrap or foam sleeves encasing the individual sample containers and lining the cooler interior. Interior packing materials should be sufficient to prevent breakage during transport. After placing individually wrapped sample containers in the cooler, fill all empty space between sample containers with padding to minimize movement against each other.
Containers for water samples should be packed in an upright position and not stacked on their sides. When shipping water samples, line the bottom of the cooler with absorbent material to contain liquids in case of breakage.
The use of frozen gel ice is convenient to maintain the samples at a temperature of less than 6°C, but water ice may also be used. When using water ice, precautions to prevent spillage from the sample cooler (i.e., containing water ice in triple bags and sealing the cooler drain) are essential to expedient delivery of the shipment to the laboratory. Line the bottom of the cooler with absorbent material when using water ice in coolers.
Immediately prior to shipment, replace the ice or frozen gel packs in the coolers so that samples will be maintained at less than 6°C during transport to the analytical laboratory.
11.4.2 SHIPPING OR DELIVERY
If utilizing a mainland laboratory, consider shipping and delivery constraints resulting from Hawaiʻi’s geographic location, especially when collecting samples with a short hold time. Ensure sample delivery to the analytical laboratory so that there is sufficient time for analysis of the constituent with the shortest hold time. Samples preserved at less than 6°C using gel packs or ice should be shipped via overnight delivery. If samples are shipped on Friday, arrange for Saturday delivery with the analytical laboratory to ensure the correct temperature is maintained throughout shipping.
Errors in shipping and delivery risk exceeding either the required sample temperatures or analysis hold times. As noted previously, results from samples exceeding the required preservation temperatures or analyzed past the hold times may or may not be usable, depending upon the DQO set forth in the SAP. If the data are deemed usable, the results from the analyses should be interpreted as minimum concentrations.
When shipping air, soil, or water samples either inter-island or to the mainland, follow all appropriate U.S. Department of Transportation (DOT) regulations, specifically Title 49 of the Code of Federal Regulations (CFR), Parts 171 through 180 (Title 49 CFR). The DOT rules and regulations apply to all samples shipped including methanol, sodium bisulfate, and nitric acid preserved samples if a commercial carrier such as FedEx transports the samples. Personnel responsible for shipping soil and water samples in Hawaiʻi should receive training and refresher training in these regulations. Specific packaging, labeling, and documentation are required by DOT regulations for most samples containing chemical preservatives. In addition, shipping dry ice is also subject to DOT regulations including, but not limited to, shipping container labeling requirements and restrictions on the weight of dry ice included in each shipping container.
Shipment of soil samples from Hawaiʻi to the mainland is also subject to United States Department of Agriculture (USDA) inspection and regulation. The USDA does not need to inspect water sample shipments. A “USDA Soil Import Permit” is required to prove that the receiving analytical laboratory is certified by the USDA to receive and properly dispose of soil. In addition, all soil sample coolers must be inspected by a USDA representative, affixed with a label indicating that the coolers contain environmental samples, and accompanied by shipping forms stamped by the USDA inspector prior to shipment (US Navy, 2007).
In Hawaiʻi, soil sample shipments are typically brought to the shipping company at the airport where the shipping company contacts a USDA representative to request an inspection. Alternatively, individuals or consulting firms may enter into an agreement with the USDA to ship soil samples. In this way, the USDA does not need to inspect each soil sample shipment. Consider USDA inspection requirements when planning sample shipments and employ custody seals on each individual sample container to ensure proper chain-of-custody control in the event coolers are opened by the USDA for inspection.
Inter-island shipment of soil in Hawaiʻi is subject to inspection and regulation by the State of Hawaiʻi Department of Agriculture (HDOA). Annual intra-state permits for shipping soil may be requested from the HDOA Plant Quarantine Branch in Honolulu.
Non-Hazardous Materials Shipment
Field personnel must state whether any sample is suspected to be a hazardous material. Samples may be shipped as non-hazardous based on previous site sample results, field screening results, or visual observations. In addition, environmental samples are currently exempt from Hazardous Goods regulations. Title 40 CFR, Part 261.40(d) states “A sample of solid waste or a sample of water, soil, or air which is collected for the sole purpose of testing to determine its characteristics or composition is not subject to this Part or Parts 262 through 267 or Part 124 of this chapter or to the notification requirements of Section 3010 of RCRA.” (Title 40 CFR) Therefore, no special regulations are required to be followed for the shipment of environmental samples from the field. Note that this provision applies to unpreserved soil and water samples (i.e., no chemical preservatives added during collection).
For groundwater samples specifically, very small quantities of certain dangerous goods may be transported without certain marking and documentation requirements as described in Title 49 CFR Part 172 (Title 49 CFR). The Hazardous Materials regulations do not apply to the commonly utilized sample preservatives methanol, sodium bisulfate, hydrochloric acid, nitric acid, sulfuric acid, and sodium hydroxide added to water samples if their pH or percentage by weight criteria is met (USEPA, 1996; USACE, 1998b; US Navy, 2007). Standard preservative volumes in standard sample containers (e.g., HCl in 40 ml volatile organic analysis [VOA] jar) fall under this definition (USEPA, 1996; USACE, 1998b; US Navy, 2007).
It is extremely important to be aware that regulations may prohibit the shipment of chemically preserved soil samples (such as methanol or sodium bisulfate preserved soil samples intended for volatile analysis). The above paragraph references the shipment of chemical preservatives in water samples only.
11.5 APPROVED ANALYTICAL METHODS
Use the methods and standard operating procedures listed in EPA SW-846 (USEPA, 1991c and 2003b) for the analyses conducted under the guidance of this TGM. Consider the analytes of interest, the sample matrices, and the minimum detectable concentrations required to accomplish project DQO when selecting analytical methods. Tables 11-A and 11-B in the Appendices provide the recommended analytical methods for soil and groundwater analysis, respectively.
Use other EPA-approved methods (such as Methods for Chemical Analysis of Water and Wastes (USEPA, 1983) for analyses that measure parameters such as pH, specific conductance, dissolved oxygen, and temperature. Document any deviations from EPA-approved methods in the SAP.
When EPA-approved methods are not available or appropriate for project-specific requirements, other recognized standard analytical methods, such as those published by the American Society for Testing and Materials (ASTM) International or the National Institute for Occupational Safety and Health (NIOSH), may be used. Example guidance documents for other methods and procedures that may be proposed for site investigations include:
- American Public Health Association (APHA), American Water Works Association, Water Environment Federation. 2005. “Standard Methods for the Examination of Water and Wastewater.” 21st Edition (APHA, 2005).
- ASTM. (updated yearly). “Annual Book of Standards.” West Conshohocken, Pennsylvania (ASTM).
- NIOSH. 1994. NIOSH Manual of Analytical Methods, Fourth Edition. Publication No. 94-113 (NIOSH, 1994).
The published methods are updated at various time intervals. Unless otherwise stated, laboratories conducting work under the guidance of this TGM will use the most current version of any specified analytical method.
On occasion, project-specific conditions might require the use of analytical methods that are either a modification of an EPA-approved method or are not an EPA-approved method. These methods will typically be provided by the laboratory performing the analysis. Any laboratory using modified EPA-approved methods or non-EPA-approved methods must provide a detailed description of sample preparation, instrument calibration, sample analyses, method sensitivity, associated QA/QC requirements, and acceptance criteria, preferably during the planning phases and the creation of the SAP. The laboratory or method developer must provide method performance study information (e.g. detection, recovery, calibration data) to confirm the performance of the method for each applicable matrix. If previous performance studies are not available, they must be developed during the project and included as part of the project results.
An example of a modification of an EPA-approved method that is important for analysis of most soil and sediment samples for metals (whether Multi-Increment samples or discrete samples) is EPA Method 3050 for preparation of metals analyses. Soil or sediment samples are typically sieved to the <2 millimeter (mm) particle size before analysis, and an analysis of particles of this size requires a minimum extraction and analysis mass of 10 grams (rather than 1 gram generally recommended in Method 3050) to reduce fundamental error in the analysis (see discussion in Subsection 4.2.2 and USEPA, 2003b; ASTM, 2003). Laboratories conducting metals analyses of soils should therefore ensure they have conducted and documented method performance data for the extraction and analysis of these larger masses of soil or sediment.
11.6 FIELD-BASED MEASUREMENT TECHNOLOGIES
Field-based measurement technologies have advanced significantly in recent years in terms of quality and utility. Field-based measurement technologies include a broad range of options including devices capable of in-situ measurements, devices capable of providing ex-situ measurements on sampled media in the field, and mobile laboratories that can be transported to the field site.
Some field methods provide qualitative or semi-quantitative analyses that may be used as screening data. A growing number of field techniques, such as field assays and field colorimetric tests, are now available that are capable of providing quantitative, analyte-specific analyses typically associated with standard fixed-laboratory techniques.
Although the HEER Office supports the use of field measurement technologies whenever available and appropriate to enhance or help speed site investigations, these technologies must be augmented by the analysis of a subset of the field samples in a standard fixed analytical laboratory. The results from samples analyzed in the field should be correlated to the results from replicate samples analyzed in the fixed laboratory to assess the precision and accuracy of the field data.
Guidance on field assays and field screening methods is presented in Section 8.
