Section 21
Ecological Risk Assessment Guidance for Coastal Marine Environments in Hawaiʻi
Interim Final – October 2018
Section 21.1 Framework for Ecological Risk Assessments
Section 21.2 Determine the Need for a SLERA
Section 21.3 Screening Level Ecological Risk Assessment
21.3.1 Preparing for a SLERA
21.3.2 Components of a Marine Sediment SLERA
21.3.3 Step 1B: Screening Level Site Characterization Data
21.3.3.1 Step 1b, Task 1. Describe Environmental Setting
21.3.3.2 Step 1b, Task 2. Compile Available Site-Specific and Reference Data on Chemicals and Endpoints
21.3.3.3 Step 1b, Task 3. Select Assessment and Measurement Endpoints
21.3.3.4 Step 1b, Task 4. Identify Complete Exposure Pathways and Potential Routes of Exposure
21.3.3.5 Step 1b, Task 5. Develop the Screening Level Preliminary Conceptual Site Model
21.3.4 Step 2: Estimating Exposure and Effects
21.3.4.1 Step 2, Task 1. Compile Screening levels
21.3.4.2 Step 2, Task 2. Calculating Contaminant Concentration(s) in Sediment and Water
21.3.4.3 Step 2, Task 3. Estimating Daily Ingested Dose to Birds and Mammals
21.3.4.4 Step 2, Task 4. Calculate Site-Specific Hazard Quotients
21.3.4.5 Step 2, Task 5. Decision Checkpoint
21.3.5 Step 3A: Refine Screening Level Default Assumptions
21.3.5.1 Step 3a, Task 1. Conduct Background Screening
21.3.5.2 Step 3a, Task 2. Evaluate Magnitude of Screening Level Exceedance and Frequency of Detection
21.3.5.3 Step 3a, Task 3. Refine Conservative Exposure Assumptions
21.3.5.4 Step 3a, Task 4. Obtain HEER Office Concurrence on Refinements
21.3.5.5 Step 3a, Task 5. Recalculate HQs using Refined Exposure Assumptions
21.3.5.6 Step 3a, Task 6. Develop SLERA Risk Characterization and Decision
21.3.6 Uncertainty
Section 21.4 Anticipating and Addressing Data Gaps
Section 21.5 Summary of Decision Logic for ERAs
Section 21.6 Baseline Ecological Risk Assessment
21.6.1 BERA Refined Problem Formulation
21.6.1.1 Sediment Dynamics
21.6.1.2 Chemicals of Potential Ecological Concern
21.6.1.3 Ecological Receptors (Assessment and Measurement Endpoints)
21.6.1.4 Refined Conceptual Site Model
21.6.2 BERA Study Design and Data Quality Objectives
21.6.2.1 Laboratory Analyses
21.6.2.2 Sediment Sampling
21.6.2.3 Pore Water Sampling
21.6.2.4 Surface Water Sampling
21.6.2.5 Biological Surveys>
21.6.2.6 Field-Collected Tissue Sampling
21.6.2.7 Toxicity Testing
21.6.2.8 Laboratory Bioaccumulation Testing
21.6.3 Data Analysis and Interpretation
21.6.3.1 Field Notes
21.6.3.2 Analytical Results
21.6.3.3 Toxicity and Bioaccumulation Tests
21.6.4 Risk Characterization
Figures
Figure 21-1 Food Chain Models Can Support Development of Conceptual Site Model
Figure 21-2 A Simple Diagrammatic Conceptual Site Model for a Rocky Intertidal Habitat with Hardbottom (such as ʻĪlio Point, Molokaʻi)
Figure 21-3 Conceptual Site Model for a Rocky Intertidal Habitat with Hardbottom
Figure 21-4 Conceptual Site Model for a Soft-Bottom Bay/Harbor Habitat (such as Hanalei Bay, Kauaʻi, or Pearl Harbor, Oʻahu)
Figure 21-5 Conceptual Site Model Prepared for a BERA at Pearl Harbor
Figure 21-6 Conceptual Site Model Focused on Exposure of a Single Receptor Group (Water Birds) to a Single COPEC (Arsenic) in Sediments and Surface Water at Waiākea Pond on Hawaiʻi Island
Figure 21-7 Conceptual Site Model Focused on a Single Class of COPECs (Energetic Compounds Associated with Discarded Munitions)
Figure 21-8A Interim Decision Logic for Sediment Investigations in Hawaiʻi
Figure 21-8B Interim Decision Logic for Sediment Investigations in Hawaiʻi (continued)
Tables
Table 21-1 SLERA Framework
Table 21-2 Components of a Marine Sediment SLERA
Table 21-3 Unique or Distinct Aquatic Habitat Types and Locations in Hawaiʻi
Table 21-4 Point Sources of Target COPECs in Hawaiʻi
Table 21-5 Assessment and Measurement Endpoints: Coastal Marine Sediments
Table 21-6 Elements of a Marine Sediment Ecological CSM
Table 21-7 HDOH HEER Office Interim Sediment Quality Guidelines for Selected Chemicals
Table 21-8. Selected Species Profiles
Table 21-9. Data Gap Analysis
Table 21-10 Questions Guiding Decision Logic for Contaminated Sediment Investigation
Table 21-11 Required, Preferred, and Optional Data for Sediment ERAs
Table 21-12 Typical Depths of Biotic Zone1
Table 21-13 Typical Tissue Volumes Required for Selected Chemical Analysis
Table 21-14 Example of Qualitative Field Notes
Table 21-15 Summary of Leptocheirus plumulosus Toxicity Test Results
Appendices
Appendix 21-A Species Profiles and Exposure/Effects Data
Appendix 21-B Scoping Checklist
Appendix 21-C Defining Ecologically-Based Decision Units
Appendix 21-D Habitat Profiles
Appendix 21-E Evaluating Bioaccumulating Chemicals
Appendix 21-F Refining Assumptions of Bioavailability
Appendix 21-G Sample Table of Contents: Baseline Ecological Risk Assessment (BERA) Work Plan/Sampling and Analysis Plan (WP/SAP) and BERA Report
Appendix Tables
Appendix 21-B
Table 21-B1 Potentially Site-related Contaminants
Table 21-B2 Potential Contaminants in Marine Habitats
Table 21-B3 Initial Sediment Screening
Table 21-B4 Initial Surface Water, Groundwater, or Pore Water Screening
Appendix 21-F
Table 21-F1 Biota-Sediment Accumulation Factors for Fish and Invertebrates
21.0 ECOLOGICAL RISK ASSESSMENT GUIDANCE FOR COASTAL MARINE ENVIRONMENTS IN HAWAIʻI
An investigation of contaminants in coastal marine and estuarine sediments in Hawaiʻi is necessarily influenced by the geophysical realities of the islands themselves and the dynamic Pacific Ocean. A brief introduction to the processes that create and redistribute sediments in Hawaiʻi provides a context for the specific guidance on conducting ecological risk assessments (ERAs) in Hawaiʻi.
The shield volcanoes that make up the main Hawaiian Islands are composed mainly of basaltic lavas. Erosion by wind and water break down these basaltic rocks into smaller particles that are transported into streams and ultimately deposited along the coast. At the same time, carbonate sediments derived from marine organisms in the surrounding waters are carried shoreward and deposited along the coast to form beaches (Fletcher et al. 2012). The processes of erosion and deposition of these two major sediment types creates a patchwork of unconsolidated substrates throughout coastal Hawaiʻi. Physical characteristics of the sediment particles, such as grain size and associated organic carbon, play a substantial role in the fate and transport, bioavailability, and toxicity of contaminants in the marine environment. These topics are introduced briefly below.
Grain size is a primary characteristic of sediment that influences the fate and transport of chemicals within the marine or aquatic environment. Geologists identify sediments by size fractions (gravel, sand, silt, and clay) and classify sediments based on the ratio of size fractions using the Wentworth grade scale (USGS 2006):
| gravel | 2 mm |
| sand | < 2 mm to > 62.5 µm |
| silt | < 62.5 µm to > 4 µm |
| clay | < 4 µm |
Geological reports typically define the top 2 cm below the sediment/water interface as surficial sediment (USGS 2006). However, standard practice in ERAs is to focus on the top 10 to 15 cm (about 4 to 6 inches), the biotic zone, where exposure of ecological receptors is greatest.
Many chemicals that cause ecological effects (such as metals, pesticides, PCBs) are known to be associated most strongly with finer-grained sediment, especially silts and clays (also called “muds”) (Morrison et al. 2011). Fine-grained sediments generally accumulate in coastal bays and other sites where wave energy is low or absent. Contaminant concentrations are expected to be highest in such depositional areas where particles smaller than 62.5 µm accumulate (NRC 1989, Grabe and Barron 2004). In contrast, sites with predominantly sand or gravel are less likely to contain toxic levels of contaminants (Morrison et al. 2011).
One of the first studies to demonstrate the importance of grain size in sediment toxicity and bioavailability evaluations focused on PCBs in coastal marine sediments on the Mediterranean coast of France. The survey documented accumulation of low chlorinated PCB congeners with the sand-size fractions (> 63 µm) and of high chlorinated congeners with the silt-size fractions (< 63 µm). Greater bioavailability and toxicity were associated with the congeners in the fine-grained sediments (Pierard et al. 1996). Later studies in coastal marine harbors in the mainland United States corroborated these findings (Ghosh et al. 2003). Concentrations of dioxins and furans (PCDD/Fs) are also known to increase as grain size decreases in marine sediments (Lee et al. 2006). However, higher chemical concentrations may not accurately represent bioavailable fractions when chemicals are bound to finer-grained sediment.
The association of PCBs with fine-grained sediments has been demonstrated in tropical habitats, as well. In a highly-contaminated marine bay in Puerto Rico, PCB concentrations were shown to be influenced not only by grain size, but also by organic content. Moreover, microbiological characteristics (biofilm, bacteria levels, and microbial community composition) acted on the PCBs to reduce chlorination levels both in deeper anoxic sediment and shallow well-oxygenated sediments (Klaus et al. 2016). Toxic levels of lead are reported to be associated with fine-grained particulates carried by certain urban streams on Oʻahu, Hawaiʻi (Hotton and Sutherland 2016).
Coastal habitats in Hawaiʻi may contain a mixture of sediment grain sizes from various sources, creating complex sediment profiles and challenging risk assessment scenarios. For example, Hanalei Bay on the north side of Kauaʻi receives fine-grained terrestrial basaltic sediment from taro fields delivered by the Hanalei River. Sand-sized sediment particles composed of calcium carbonate from nearshore coral reefs are transported into the bay by wave action. The Hanalei River carries so much suspended sediment that it often exceeds federal water quality standards for turbidity (Takesue et al. 2009). Despite the dominance of fine-grain sediments near the river mouth, organochlorine pesticides, PAHs, and metals were detected in sediment at very low levels. Concentrations of organic chemicals in Asian clam (Corbicula fluminea), giant mud crab (Scylla serrata), and ʻakupa sleeper fish (Eleotris sandwicensis) were also below ecological effect levels (Orazio et al. 2010). In contrast, sediment pore water was toxic to sea urchin fertilization (but not development) in clay and mud samples near the river mouth (Carr and Nipper 2007; Cochran et al. 2007). Further complicating the interpretation of ecological risk at this site is the seasonal influence of waves, which can flush out the finer-grained sediment from the bay during winter storms (Takesue et al. 2009).
The studies in Hanalei Bay illustrate the difficulty of drawing conclusions about ecological risk from a single line of evidence. Concentrations of chemicals in sediment of different grain sizes, surface water, pore water, and biota may all contribute to risk, but no single measure can adequately characterize the site. Actual exposure of ecological receptors to contaminant in sediment is influenced by both the presence and bioavailability of contaminated sediment and the absence of wave energy that removes sediment from the site. Although substantial deposition of fine-grained terrestrial sediment containing contaminants could indicate potential ecological risk, the regular winter flushing at this site reduced the risk to acceptable levels (Orazio et al. 2007, Takesue et al. 2009).
Beaches are eroding across Hawaiian Islands that have been evaluated (Kauaʻi, Oʻahu, Maui) more than accreting (Fletcher et al. 2012) and coastal erosion is expected to nearly double over the next few decades across areas studied, except Kailua Beach on Oʻahu. Nevertheless, sediment dynamics are spatially variable, and areas of erosion and accretion may be separated by only a few hundred meters. Each small embayment created by rocky headlands is influenced by local wave energy and terrestrial processes, creating a patchwork of erosion and accretion along the shore. The most recent data on coastal erosion and accretion of shorelines on Kauaʻi, Oʻahu, and Maui are available at (Fletcher et al. 2012). This USGS information should be consulted during the site characterization phase of the SLERA (See Subsection 21.3.3).
Data on grain sizes are site-specific; there is no comprehensive assessment for the state, as grain size on beaches changes seasonally due to wave energy. Most beaches are sand and thus less conducive to adsorbing contaminants compared with finer-grained silt and clay fractions (Storlazzi, 2016), personal communication). The risk assessor should review available data on grain size at the site. If grain size has not been adequately characterized at the site (considering season and specific location), data collection should be considered prior to initiating an ERA. If the site is predominantly sand, the need for conducting additional chemical characterization in the area should be evaluated. Based on the CSM, additional chemical characterization may or may not be necessary. If the site has a patchy distribution of grain sizes, chemical characterization should focus on areas where silts and clays are dominant.
The HEER Office ERA program for marine coastal environments provides guidance for conducting screening level ERAs (SLERAs) and Baseline ERAs (BERAs) in these coastal habitats. Alternative approaches or methods to the guidance provided in this section may be acceptable but should be discussed with the HEER Office for approval. The ERA program is process-oriented in that a site progresses only as far as required by the site-specific characteristics. The level of effort devoted to preparing and submitting information to the HEER Office is determined by the level of risk posed by the site. A site may exit the process at any of several points marked by management decisions and supported by technical analysis.
An ERA at a marine sediment site typically begins as a SLERA, and then may proceed to a more site-specific and in-depth BERA, if necessary. In many cases, the ERA will be conducted as part of a larger site investigation, although some sites may be addressed as strictly ERA sites. In both instances, the overall approach to conducting an ecological site investigation should generally be consistent with guidance elsewhere in the TGM, particularly in the following sections:
- TGM Section 3: Site Investigation Design and Implementation
- TGM Section 4: Decision Unit Characterization
- TGM Section 5: Field Collection of Soil and Sediment Samples
This ERA guidance is specific to the tropical marine environment of Hawaiʻi, but draws on decades of technical development of ERA methods by federal agencies in the U.S. and their counterparts in Australia and New Zealand, individual state agencies, independent researchers, and universities. This guidance combines the widely-used U.S. EPA framework, which provides a logical step-wise approach to conducting ERAs, with a more regionally focused approach suitable for tropical marine ecosystems. The HEER Office has developed this regionally-focused guidance to efficiently evaluate exposure and effects using Hawaiʻi-specific receptor and toxicity data wherever it is available. Readily available ecological exposure and effects data for 22 marine species in Hawaiʻi are compiled in this guidance (see Appendix 21-A). As additional ERAs are prepared and more Hawaiʻi-specific data become available, the on-line ERA TGM guidance will be updated to fill data gaps and refine exposure and effect default values and assumptions.
The HEER Office assumes that consultants and risk assessors using this guidance are familiar with the concepts and terminology of ERAs. Complete citations for references cited in this ERA guidance are provided in Master Reference List. Appendices to this guidance contain additional information, as follows:
- APPENDIX 21-A SPECIES PROFILES AND EXPOSURE/EFFECTS DATA
- APPENDIX 21-B ERA SCOPING CHECKLIST
- APPENDIX 21-C DEFINING ECOLOGICALLY-BASED DECISION UNITS
- APPENDIX 21-D HABITAT PROFILES
- APPENDIX 21-E EVALUATING BIOACCUMULATING CHEMICALS
- APPENDIX 21-F REFINING ASSUMPTIONS OF BIOAVAILABILITY
- APPENDIX 21-G CONTENTS OF A BERA WP/SAP AND BERA REPORT
The risk assessor is responsible for providing technical justification for the methods and assumptions that underlie the ERA. All references cited in the ERA must be made available for review by the HEER Office upon request. The HEER Office maintains a large library of peer-reviewed literature and government reports that may be useful to the risk assessor. Close coordination with the HEER Office will provide opportunities to share references and ensure that the most current useful information is available throughout the ERA process.
21.1 FRAMEWORK FOR ECOLOGICAL RISK ASSESSMENTS
An ERA is a qualitative and/or quantitative appraisal of the actual or potential effects of one or more chemicals on plants and animals in the wild. At its simplest, risk can be defined as a function of the overlap in space and time of a stressor (a chemical) and a living organism (a receptor) where the stressor causes some adverse effect on the receptor. The process of risk assessment is designed to (1) identify the distribution and magnitude of chemical stressors; (2) identify the locations of living organisms that are sensitive to the chemical stressor; and (3) quantify the probability that the receptor will be exposed to the stressor and experience adverse effects related to the exposure.
This simple model of spatial and temporal overlap of a chemical and an organism is rarely encountered in the field, however. Instead, ERAs must often address sites where multiple chemicals have been released into several media (soil, groundwater, sediment, surface water) and numerous receptors are potentially exposed during all or part of their complex life cycles. Chemicals may be present but physically bound to media so that they do not exert a toxic effect on organisms. Concentrations of chemicals in background/ambient/reference samples may confound the interpretation of risk at the site. Information on the sensitivity of local organisms to the chemicals at the site may be unavailable. These and other difficult issues make the ERA process complex and add to the uncertainty of decisions based on ERA results.
In an effort to strengthen and streamline the ERA process, USEPA published an 8-step framework for ERAs that has been widely adopted, with modification, by national and state programs around the world (USEPA 1992e). Steps 1and 2 of the USEPA framework, generally referred to as the SLERA, are primarily based on limited site-specific sediment data and default assumptions about exposure and effects. Oftentimes, the SLERA incorporates the initial part of Step 3 (commonly referred to as Step 3a) in which the conservative default assumptions of Steps 1 and 2 are refined to focus the ERA process on the chemicals and receptors of greatest concern at the site. This HEER Office guidance includes Step 3a in the SLERA.
In addition to the USEPA framework, information and technical advances from the Australian/New Zealand Environment and Conservation Council / Agriculture and Resource Management Council of Australia and New Zealand (ANZECC/ARMCANZ) Guidance on ERAs will continue to be evaluated as they pertain to tropical marine sediments. Tables and data in the HEER Office TGM will be updated periodically as new information becomes available from sources relevant to tropical marine environments.
The risk assessor should realize that preparing an ERA is seldom a simple or linear process. More often, the risk assessor will work with data from many disciplines, including geologists, hydrologists, toxicologist, ecologists, and chemists to develop an understanding of the unique situation at the site. Some of the required elements may be available to the risk assessor from the start, while others may prove to be unobtainable within the time frame of the investigation. The steps and tasks can be approached in a different order; some processes may run concurrently and some may be repeated as the need for additional information becomes apparent. The risk assessor should maintain communication with the HEER Office and seek confirmation and clarification on the chosen approach whenever necessary.
21.2 DETERMINE THE NEED FOR A SLERA
An ERA is not required at every site where a release of chemicals has occurred. Sites where no ecological habitat exists or exposure pathways are incomplete are not required to be evaluated for ecological risk. Areas of coarse-grained sediment and high wave energy may require little or no investigation (see ERA Scoping Checklist in Appendix 21-B). To determine the need for an ERA, a person familiar with the site should complete the ERA Scoping Checklist (Appendix 21-B). The checklist is designed to help the risk assessor characterize the ecological setting of the target site and to identify complete and potentially important ecological exposure pathways. The checklist guides the risk assessor through the process of identifying relevant documents and organizing available information on the need for an ERA, referring to subsections of this HEER Office ERA Guidance when necessary. The ERA Scoping Checklist should be completed early in the investigation process to support a determination on the need for a SLERA at the site.
The preparer submits the ERA Scoping Checklist to the HEER Office for review. The HEER Office confirms that the checklist is complete and recommends future action, if warranted. If the ERA Scoping Checklist indicates that the site is excluded from ERA requirements, no other action is necessary. If the ERA Scoping Checklist indicates that exposure pathways are potentially complete and ecological habitat may be affected, then the risk assessor should initiate a SLERA in accordance with this guidance.
Sites where a potential ecological risk occurs may be evaluated using a SLERA, a BERA, or both. Most sites begin with the SLERA, although this is not strictly required. If the risk assessor believes that site conditions are relatively certain to warrant a BERA, then it is not necessary to conduct a separate SLERA. The conceptual elements of a SLERA will ultimately be incorporated into the BERA, but skipping the SLERA steps can save the risk assessor time and effort that can be better dedicated to the BERA. The risk assessor should consult with the HEER Office to obtain agreement on such an approach before initiating a BERA.
21.3 SCREENING LEVEL ECOLOGICAL RISK ASSESSMENT
Unlike the ERA Scoping Checklist, which can be completed by anyone familiar with the site, the SLERA should be prepared by a person or a team with knowledge of the chemicals, receptors, exposure pathways, and other ERA elements necessary to the investigation.
The purpose of the SLERA is to focus investigation and remediation on sites and chemicals that may pose an unacceptable risk to ecological receptors. The SLERA provides an opportunity for a site to exit the ERA Program with a minimum of effort if the site truly poses very little or no risk to ecological receptors. In cases where the entire site cannot be shown to pose a level of risk below applicable screening levels or alternate (approved) decision level based on a more a detailed evaluation, selected chemicals or receptors may still be identified for possible elimination from further investigation in later steps of the ERA.
21.3.1 PREPARING FOR A SLERA
If the ERA is being conducted as part of a larger site investigation, data collected for other purposes may be available to initiate the SLERA, as shown in Step 1b (Table 21-1). For example, sites where a chemical release happened some time ago may have been investigated for risk to human health. Sites where a discrete release of chemicals occurred may have been subjected to emergency removal actions and/or an investigation of residual risk. In such cases, the risk assessor should gather all available data from the site in preparation for the SLERA. Note that the existence of data from an umbrella investigation does not necessarily mean that no additional samples will be required. Available site-specific data are reviewed for usability during Step 1b and the need for additional data to adequately characterize current site conditions is determined. The risk assessor is encouraged to consult with the HEER Office if unsure about the need for additional data collection. Of special concern is the potential need for additional data collection in cases where existing data were based on a small number of discrete samples, which are not likely to be representative of the decision unit (see TGM Section 4). On the other hand, the risk assessor may have access to additional site-specific data not typically required for a SLERA (such as field-collected tissue samples). In such cases, the additional data can certainly be used in the SLERA to support a decision on the need for further investigation (see Step 2).
If the SLERA is being conducted outside the context of a larger investigation, then some additional steps will be necessary to initiate development of data collection suitable to support a SLERA. (Step 1a, Table 21-1). Guidance on conducting a general site investigation is provided in Sections 3.0, 4.0, and 5.0 of the TGM. Specifically, any field sampling and analysis plan (SAP) should be prepared in accordance with the decision unit (DU) and Multi Increment sampling (MIS) approach described in the TGM. Additional guidance on defining DUs for ERAs is in Appendix 21-C.
The risk assessor should review the pertinent subsections of the TGM, then consult with the HEER Office for assistance in developing a SAP that satisfies the requirements of a SLERA.
Table 21-1. SLERA Framework
| Step 1A: Develop and Implement Screening Level Sampling and Analysis Plan (if available data are not adequate to support a SLERA) | |
| Only as Needed: Activities: If site-specific data are not available, prepare a sampling and analysis plan (SAP) in accordance with site investigation guidance in Sections 3, 4, and 5 of the TGM, including clear data quality objectives (DQO). Once data are available, complete the outputs in Step 1B and then proceed to Step 2 below |
Outputs:
|
| Step 1B: Screening Level Site Characterization Data and Ecological Effects Evaluation | |
| Activities: Task 1-1: Describe environmental setting (location, habitats, expected species, sources of chemicals, previous investigations). Task 1-2: Compile available site-specific and background, ambient, and reference analytical data (from ERA Scoping Checklist or other sources); include a description of ecotoxicity and bioaccumulative potential of target chemicals. Task 1-3: Select assessment and measurement endpoints (see USEPA 1996za; 2016b). Task 1-4: Identify exposure pathways and ecological receptors. Task 1-5: Develop preliminary CSM. |
Outputs:
|
| Step 2: Estimate Preliminary Exposure Concentrations and Calculate Hazard Quotients | |
| Activities: Task 2-1: Compile screening levels for all media in your dataset. Sediment quality guidelines (SQG) are in Table 21-7. Surface water, groundwater, and sediment pore water should be screened against HEER Office Environmental Action Levels (EALs) for aquatic toxicity (aquatic habitat goals), surface water, and/or groundwater, as applicable and if included in this guidance (see HEER Office EAL Surfer tool). USEPA National Water Quality Criteria (USEPA 2016a, or current reference) can be referenced for chemicals not included in HEER Office EALs (if available). Tissue concentrations may be compared with critical body residues (CBR) reported in the literature). Toxicity reference values (TRV) for receptors evaluated through food chain modeling (e.g. mammals and birds) may be derived from published studies and reports. Task 2-2: Estimate average exposure concentrations that are representative for sediment and/or water decision units at the site (see TGM Sections 3, 4, and 5). Task 2-3: Calculate daily dose for higher trophic level receptors (birds and mammals). Task 2-4: Calculate hazard quotients (HQ) using representative DU-MIS concentrations for sediments/no effect screening levels, representative pore water or surface water concentrations/no effect screening levels, or maximum tissue concentrations to calculate daily doses for comparison with low TRVs. Task 2-5: Summarize HQs, identify chemicals of potential ecological concern [COPEC], and make a decision about the site. If risk is potentially unacceptable, continue to Step 3A), otherwise the ERA process can stop. |
Outputs:
|
| Step 3A: Refine Screening Level Default Assumptions | |
| Activities: Task 3-1: Compile available data representing background, ambient, or reference concentrations and submit to the HEER Office for concurrence. Compare the site sediment and/or water concentrations with background, ambient, and reference concentrations, as available. Task 3-2: Evaluate the magnitude of exceedance, frequency of detection, and distribution of exceedances in sediment (and water, if appropriate) at the site to determine whether any chemicals should be eliminated as COPECs. Task 3-3: Confirm that the data used are reasonably representative for decision units at the site. Evaluate the reasonableness of default conservative exposure assumptions (100 percent bioavailability of chemicals, 100 percent site use by receptors, maximum chemical concentrations, etc.) and adjust assumptions (if appropriate). Consider the influence of geophysical and geochemical parameters such as grain size, total organic carbon, pH, and other factors on bioavailability of chemicals. If the area is known to be erosional, consider the short-term and long-term fate of contaminated sediments. Task 3-4: Confirm with HEER Office that the Step 3a refinements are technically defensible based on site conditions. Task 3-5: Recalculate HQs using more realistic representative exposure concentrations. Task 3-6: Summarize HQs, evaluate uncertainty, and develop risk characterization to support a decision about the site. If risk is potentially unacceptable, continue to the baseline ERA (BERA); if not, the ERA process can stop. |
Outputs:
|
21.3.2 COMPONENTS OF A MARINE SEDIMENT SLERA
In the interest of streamlining the SLERA process and promoting consistency among SLERAs, the HEER Office provides examples or templates for many of the common components of a SLERA. Additional examples/templates will be added to this TGM as they are developed. Return to the Top of the Page
| Table 21-2. Components of a Marine Sediment SLERA | |
| Required Information | Source of Information |
| Representative concentrations of chemicals in sediment from the site | Risk assessor (representing site owner/regulated community) compiles available site-specific data. |
| Sediment Quality Guidelines (SQG), HEER Office EALs and background/ambient/reference concentrations | HEER Office provides SQGs for most target chemicals (See Table 21-7); HEER Office provides EALs for aquatic toxicity, surface water, and groundwater (see EAL surfer; risk assessor supplements as needed. |
| Potential receptors (identified by habitat or exposure guild) | HEER Office provides species profiles and exposure/effects data (Appendix 21-A, habitat profiles (Appendix 21-D); risk assessor selects and augments as necessary. |
| Conceptual Site Model (CSM) (identifying pathways and representative receptors) | HEER Office provides examples for several habitats (Figures 21-2 through 21-7); risk assessor customizes to site and supplements when necessary. |
| Sediment dynamics (erosional or depositional) | Risk Assessor provides, based on US Geological Survey reports (Fletcher et al. 2012) or site-specific data |
| Toxicological profiles for COPECs | Risk Assessor provides; HEER Office may assist with reference materials. |
| Exposure factors for assessment endpoint receptors | HEER Office provides examples for some common receptors; risk assessor supplements as necessary (Appendix 21-A). |
21.3.3 STEP 1B: SCREENING LEVEL SITE CHARACTERIZATION DATA
The screening-level site characterization, known as preliminary problem formulation, serves as an organizing foundation for the SLERA. It incorporates physical, chemical, and biological elements and features of the site that will guide the ERA process. Although each site is different, Step 1B usually includes five tasks, which are introduced below and discussed in more detail in the subsections below:
- Describe environmental setting (location, habitats, expected species, sources of chemicals, previous investigations) and summarize results of previous investigations [Step 1B, Task 1]
- Compile available site-specific, background, ambient, and reference analytical data (from ERA Scoping Checklist or other sources); include a description of ecotoxicity and bioaccumulative potential of target chemicals [Step 1B, Task 2]
- Select assessment and measurement endpoints [Step 1B, Task 3].
- Identify exposure pathways and receptors [Step 1B, Task 4]
- Develop preliminary CSM, [Step 1B, Task 5]
21.3.3.1 STEP 1B, TASK 1; DESCRIBE ENVIRONMENTAL SETTING
The environmental site setting includes a description of the location, habitats, expected species, sources of chemicals, and other site-specific information pertinent to the SLERA. The site setting should be based on information gathered during a site visit and/or readily available information.
The HEER Office has compiled a list of habitat types (see Table 21-3) and more detailed information on several key habitat types in Hawaiʻi (see Appendix 21-D) to aid in developing the environmental setting and help foster consistency in ERAs across the state. Additional habitat profiles will be provided under subsequent phases of guidance development.
Habitat information in Appendix 21-D should be augmented by the following site-specific information whenever possible:
- Physical description of the site including:
- Size (acres)
- Potentially affected habitats (mudflats, coral reefs, seagrass beds, etc.) [Include map or figure of location and habitat types.]
- Sediment type or grain size distribution (coral rubble, coarse sand, silt, etc.)
- Wave environment (high energy, low energy, protected harbor, etc.)
- Salinity, tidal range (intertidal, subtidal), bathymetry, etc.
- Erosional/Depositional area (see Fletcher et al. 2012)
- Current and historical uses of the site (known or suspected)
- Potential ecological receptors present at the site (per habitat within site)
- Surrounding land use
- Any potential sources of contaminants not related to the site activities (storm water outfalls, stream discharge, nearby industries, recreational vessel traffic, etc.)
- Known or suspected threatened and/or endangered species or other protected species/habitats within or adjacent to the site
- Maps, photographs, and figures of the site (current and historical)
- Any site-specific studies conducted at the site or in adjacent habitats
A habitat is considered important if it comprises a substantial portion of the site or provides high-value areas for target receptors. Provide as much detail as is available about the relative distribution of habitats within the site. For example, at a site that is 90 percent soft-bottom and 10 percent coral rubble covered with algae, both soft-bottom and algae-covered rubble would be included as important habitat types. The soft-bottom is spatially dominant and the algae-covered rubble provides sheltering and foraging habitat likely to be used disproportionately by some receptors.
Return to the Top of the Page
| Table 21-3. Unique or Distinct Aquatic Habitat Types and Locations in Hawaiʻi | |
| Habitat Type | Description/ Example Locations |
| Mudflats/Coastal Wetlands/Lagoon (Appendix 21-D) | Significant mudflats occur in Māmala Bay, Pearl Harbor, and Kāneʻohe Bay |
| Rocky Intertidal and Tidepools (Appendix 21-D) | Rocky intertidal habitat dominates most shorelines of all islands where constant wave action, currents, steep submarine slopes, and a lack of offshore sand reservoirs limit the accumulation of sand. ʻĪlio Point on Hawaiʻi is a typical high-energy tidepool habitat. |
| Coastal Fishponds (Appendix 21-D) | Māmala Bay, Pearl Harbor, several around Kāneʻohe Bay, and three on the southwestern coast of Kauaʻi. |
| Seagrass Beds (Appendix 21-D) | Significant seagrass beds are known from the inner reef flats of south Molokaʻi; ʻAnini (Kauaʻi); near Māmala Bay and Kāneʻohe Bay; others exist but are not mapped |
| Mixed Sediment Bays and Harbors (Appendix 21-D) | Pearl Harbor; soft sediment overlaid on limestone platform of fossil reef origin; soft sediments often composed of carbonate grains derived from coralline algae, coral, mollusk fragments, foraminiferans, and tests of bryozoans and echinoderms |
| Young Volcanic Substrate; Little Sediment (profile not yet complete) | Big Island |
| Deep Channels (profile not yet complete) | ʻAlenuihāhā Channel, between Hawaiʻi and Maui |
| Soft Sediment Bays (profile not yet complete) | Hanalei Bay, Kauaʻi; no coral rubble |
| Sandy Beach (profile not yet complete) | Along the lagoon reaches of atoll islets and especially along the west and south sides of Kauaʻi, Oʻahu, Molokaʻi, Maui, Lānaʻi, and Hawaiʻi; also along bays and coves on mature islands |
| Anchialine Pools (profile not yet complete) | Rocky shorelines on most islands, up to several hundred meters inland; The Kaloko-Honokohau Park on the western coast of Hawaiʻi contains about 10% of Hawaiʻi’s anchialine ponds. |
| Stream-fed Estuarine Wetlands (profile not yet complete) | Māmala Bay and Kāneʻohe Bay, Oʻahu |
| Mangroves (Introduced) (profile not yet complete) | In addition to invading coastal fishponds (see above), mangroves have spread to mud flats and estuarine waters around most of the Islands and to some rocky coastal areas around Hawaiʻi Island. |
| Subtidal Hardbottom (profile not yet complete) | Hardbottom occurs on every island; shallow benthic communities occur in depths of up to 50 meters or more, on basalts, and on consolidated limestone (reef carbonates, beach rock). The distribution of benthic communities is determined by light penetration, temperature, wave action, availability of substrate, and movement and accumulation of sediments. |
| Coral Reef (profile not yet complete) | About 80% of coral reef habitat in Hawaiʻi is in the Northwest Hawaiian Islands (NWHI), including atolls, islands, and banks. The high volcanic islands of the Main Hawaiian Islands (MHI) typically include non-structural reef communities, fringing reefs, and two barrier reefs (Kāneʻohe Bay and Moanalua Bay, Oʻahu). |
Species at the Site
Species at the site should be grouped into two categories: (1) typical or common species and (2) threatened, endangered, or specially protected species. A list of typical or common species can be generated using Hawaiʻi-specific publications and websites cited throughout this guidance. Profiles of select species are in Appendix 21-A.
Information on threatened, endangered and otherwise protected species and habitats is widely available on websites published by state and federal resource agencies. The status of species and habitats may change over time. The risk assessor should check the websites below, and other websites, as necessary, to make sure the most current information is used in the ERA:
- The Hawaiʻi Department of Land and Natural Resources 700-page review, Hawaiʻi’s Comprehensive Wildlife Conservation Strategy, describes habitats, species, and threats across the MHI and NWHI (Mitchell et al. 2005). This document lists and describes the distribution and abundance of species of “greatest conservation need,” and provides locations and relative condition of key habitats; threats to species; conservation actions proposed; and plans for monitoring species and their habitats. Fact sheets address larger taxa or groups relevant to the marine ERA program, including waterbirds, seabirds, migratory shorebirds and waterfowl, anchialine pond fauna, marine mammals, marine reptiles, marine fishes, and marine invertebrates.
- Species Recovery Plans, critical habitat designations, and 5-Year Status Reviews provide extensive information on life history and habitat requirements, as well as current threats to the species protected under the Endangered Species Act (ESA). Recovery plans for species under the jurisdiction of U.S. Fish and Wildlife Service (FWS), such as coastal birds, are available at (USFWS 2018). See (USFWS 2018b) for links to documents proposing and designating critical habitat for FWS species. Links to 5-Year Status Reviews are on the species profile page for each species.
- The National Oceanic and Atmospheric Administration, Pacific Islands Regional Office of the National Marine Fisheries Service (NOAA 2018) provides information on ecological resources including protected species and unique habitats.
- The U.S. Navy has compiled data on Hawaiian species in the following documents:
Identify Potential Sources of Contamination
The site-specific data compilation activities of the SLERA should identify contaminants potentially present at the site and the sources of those contaminants based on the types of activities known or suspected to have taken place at the site. Typical point sources and COPECs are compiled in Table 21-4. While the information in Table 21-4 can be used as a starting point, it should not be assumed that these are the only chemicals associated with site activities. Activities specific to a particular facility may have resulted in different and/or additional chemicals being released into the environment. Also, because operations often change at a site over time, a thorough search of the site history is needed to determine which chemicals may be present at the site.
| Table 21-4. Point Sources of Target COPECs in Hawaiʻi | |||
| Type of Point Source | Chemicals | Example Locations | Documents |
| Harbors and marinas | Antifouling compounds (Irgarol and other copper-based compounds); polycyclic aromatic hydrocarbons (PAHs) | Ala Wai Marina, Kāneʻohe Bay Yacht Club, Kāneʻohe Bay Makani Kai Marina, Sand Island Keʻehi Marina, Waikīkī Yacht Club | Knutson et al. 2012 |
| Former military installations or disposal sites | Metals, polychlorinated biphenyls(PCBs), munitions (energetics), pesticides | Waiʻanae, Oʻahu; Mākua Military Reservation, Oʻahu; Midway Atoll, Sand Island | Garcia et al. 2009; USACE 2012; Tetra Tech 2009; Taylor et al. 2009 |
| Long Range Navigation (LORAN) stations | PCBs, lead | Kure Atoll, Cocos Island, Guam; ʻĪlio Point, Molokaʻi; Tern Island, French Frigate Shoals | Element Environmental 2009; Element Environmental 2010; ESI 2012; USCG 2000; Woodward-Clyde Consultants 1994 |
| Shipyards | Tributyltin (TBT), antifouling paints, copper, zinc | Pearl Harbor, Oʻahu | Grovhoug 1992 NAVFAC 2007 |
| Former shooting ranges on coast | Lead shot | ||
| Estuaries | Metals, PAHs, pesticides, pharmaceuticals, polybrominated diphenyl ethers (PDBE), pathogens, PCBs | Grovhoug 1992NAVFAC 2007 | |
| Sugar mill or canec manufacture dumping areas | arsenic, herbicides | Waiākea Mill Pond, Wailoa River | Hallacher et al. 1985 HDOH 2005c |
| Urban/ storm drains | PAHs | Various streams, Oʻahu | Zheng et al. 2011 |
| Urban/ storm drains | Metals | Nuʻuanu watershed, Oʻahu | Andrews and Sutherland 2004 |
| Urban Run-off | Microbial and nutrients | Hanalei Bay, Kauaʻi | Boehm et al. 2011 |
| Urban Run-off | Pesticides and metals | Various locations in Oʻahu and Kauaʻi | Brasher and Wolf 2007 |
| Agricultural Run-off | Pesticides | Pineapple fields; Honolua Stream entering Honolua Bay, Maui | |
| Agricultural Run-off | Arsenic, herbicides, pesticides | Island of Hawaiʻi sugar cane plantation | Cutler et al. 2013 |
| Agricultural Run-off | Pesticides | Taro ponds; run-off to Hanalei River, Kauaʻi | DLNR DAR 2012 |
| Golf courses | Herbicides; pesticides | ||
| Sewage outfalls | Metals, PAHs, pharmaceuticals, pathogens | ||
| Sediment disturbance | |||
| Coastal marine construction sites | All chemicals associated with sediment in given location | ||
| Dredging | All chemicals associated with sediment | Kūhīo and Hilo Bays, Hilo Commercial Harbor, Hawaiʻi Island | USACE 2008 |
| Shoreline erosion (landfill) | All chemicals associated with sediment; solid waste in landfills exposed to water and air | ||
21.3.3.2 STEP 1B, TASK 2; COMPILE AVAILABLE SITE-SPECIFIC AND REFERENCE DATA ON CHEMICALS AND ENDPOINTS
Step 1b, Task 2 requires the risk assessor to compile available site-specific and reference analytical data (from ERA Scoping Checklist or other sources), evaluate ecotoxicity screening levels, and identify bioaccumulative chemicals.
All analytical data collected at the site during current or previous investigations should be compiled and evaluated for use in the SLERA. Analytical data more than five years old may no longer be representative of site conditions and should be discussed with the HEER Office.
A list of site-related chemicals compiled during the scoping phase (see Subsection 21.2 and Appendix 21-B, Table B-1) will be evaluated in the SLERA. Chemicals that act primarily through direct toxicity are evaluated using a hazard quotient (HQ) approach in Step 2. Chemicals that are known or expected to bioaccumulate in living organisms are also evaluated separately because sediment and water screening levels do not typically incorporate risk due to bioaccumulation in tissues (see Appendix 21-E).
Site-specific and reference data compilations for the SLERA should describe the direct toxicity and bioaccumulation potential of COPECs at the site. Direct ecotoxicity of COPECs in sediment is evaluated by comparison of sediment concentrations with SQG designed to be protective of benthic invertebrates in direct contact with sediment (see Subsection 21.3.4 and Table 21-7). In the SLERA, the ecotoxicity evaluation may focus on groups of chemicals such as organochlorine pesticides, as opposed to specific pesticides. The risk assessor may augment the HEER Office SQG in Table 21-7 with data from the published literature to develop ecotoxicity profiles for COPECs whose primary mode of action is direct toxicity. HEER Office EALs (screening levels) for aquatic habitat goals, surface water, and groundwater can be referenced and used for data evaluation, as applicable. See the detailed table links in the EAL surfer tool for breakdown of the aquatic habitat goals and surface water EALs by marine, estuarine, or freshwater categories.
Separate from direct toxicity, some chemicals bioaccumulate in living organisms, meaning that they contain higher concentrations of a chemical in their tissues than in surrounding sediment or water. When bioaccumulated chemicals are transferred from one organism to another through the food web, the concentration may increase even more, in a process called biomagnification. Bioaccumulation of chemicals in tissues provides a pathway for chemicals to transfer to on-site and off-site receptors. The concentration of a bioaccumulating chemical in sediment may be considered safe for receptors in direct contact with sediment but not for receptors higher on the food web. Therefore, bioaccumulative chemicals require additional evaluation in the SLERA to determine whether they pose adverse risks to higher trophic levels that are not addressed by the SQGs.
21.3.3.3 STEP 1B, TASK 3; SELECT ASSESSMENT AND MEASUREMENT ENDPOINTS
A key task of the SLERA site characterization process is to identify the ecological resources to be protected at the site (known as assessment endpoints) and the measures used to evaluate risks to those resources (known as measurement endpoints or measures of effect). Assessment endpoints are explicit expressions of the environmental value that is to be protected. The selection of these endpoints is based on the habitats present, migration pathways of probable contaminants, and relevant exposure routes for the receptors. Suitable assessment endpoints species are characterized as follows:
- ecological relevance;
- susceptibility to known or potential stressors; and
- relevance to management goals (USEPA 1998).
For additional discussion of the selection of proper assessment endpoints, see the following:
- Generic Ecological Assessment Endpoints (GEAEs) for Ecological Risk Assessment: Second Edition with Generic Ecosystem Services Endpoints added. (USEPA 2016b)
- Guidelines for Ecological Risk Assessment (USEPA 1998i)
- ECO Update: Identify Candidate Assessment Endpoints Ecological Significance and Selection of Candidate Assessment Endpoints (USEPA 1996za)
Measurement endpoints are estimates of quantifiable biological features or processes (such as mortality, growth, and reproduction) that are believed to be linked to meaningful effects on the assessment endpoints selected at the site.
Assessment endpoints selected for the SLERA are typically carried through to the BERA, unless it is discovered during the SLERA that the species does not fit the requirements of an assessment endpoint (it is not present, not exposed to contaminated media, not valued by the community, or eliminated during earlier steps in the SLERA). Measurement endpoints selected for the SLERA are often augmented in the BERA by endpoints more focused on particular chemicals or pathways of interest at the site.
Example preliminary assessment and measurement endpoints for a coastal marine sediment site in Hawaiʻi are in Table 21-5. Measurement endpoints for the SLERA and the BERA are shown to illustrate the differences between the two phases of an ERA.
| Table 21-5. Assessment and Measurement Endpoints: Coastal Marine Sediments | |||
| Ecological Guild | Assessment Endpoint | Typical Species | Measurement Endpoint |
| Seaweed (Limu) | Organism Level: Survival, growth, and reproduction
Population/Community Level: Distribution and abundance within DU |
|
SLERA:
|
BERA: Concentrations of chemicals in composite samples of tissues collected from the DU or estimates of tissue concentrations from sediment using BSAFs compared with tissue effect levels for marine algae (Tissue effect levels identified through literature review).
|
|||
| Soft-bodied benthic invertebrates (macroinfauna) | Organism Level: Survival, growth, and reproduction
Population Level/Community Level: Distribution and abundance within DU |
|
SLERA: Concentrations of chemicals in site MIS sediment samples compared with SQG protective of polychaetes. |
BERA:
|
|||
| Stony Corals | Organism Level: Survival, growth, and reproduction (of colony)
Population/Community Level: Distribution and abundance within DU |
|
SLERA: Concentrations of chemicals in site MIS sediment samples compared with SQG protective of corals. |
BERA:
|
|||
| Epibenthic Invertebrate (macrofauna ) | Organism Level: Survival, growth, and reproduction
Population/Community Level: Distribution and abundance within DU |
|
SLERA:
|
BERA (Echinoderm only): Laboratory toxicity test of effect of exposure to sediments and/or sediment pore water on sea urchin survival and development. BERA (Other macrofauna):
|
|||
| Benthic Fish (herbivores, corallivores, carnivores) | Organism Level: Survival, growth, and reproduction
Population Level: Distribution and abundance within DU |
|
SLERA:
|
BERA:
|
|||
| Pelagic Fish (piscivores) | Organism Level: Survival, growth, and reproduction
Population Level: Distribution and abundance within DU |
|
SLERA: No direct link to sediment. Assume food web link to lower trophic levels in the DU. |
BERA:
|
|||
| Sea turtles | Organism Level: Survival, growth, and reproduction
Population Level: Distribution and abundance within DU |
|
SLERA: Conservative estimate of daily ingested dose of contaminant within DU compared with no observed adverse effect level (NOAEL) TRVs for sea turtles (or surrogate reptiles). (TRVs identified through literature review). |
| BERA: Realistic estimate of daily ingested dose of contaminant within DU compared with lowest observed adverse effect level (LOAEL) TRV for sea turtles (or surrogate reptiles). TRVs identified through literature review. | |||
| Piscivorous birds | Organism Level: Survival, growth, and reproduction
Population Level: Distribution and abundance within DU |
|
SLERA: Conservative estimate of daily ingested dose of contaminant within DU compared with NOAEL TRV for piscivorous seabirds (or surrogate birds). (TRVs identified through literature review). |
| BERA: Realistic estimate of daily ingested dose of contaminant within DU compared with LOAEL TRV for piscivorous seabirds (or surrogate birds). TRVs identified through literature review. | |||
| Marine mammals | Organism Level: Survival, growth, and reproduction
Population Level: Distribution and abundance within DU |
|
SLERA: Conservative estimate of daily ingested dose of contaminant within DU compared with NOAEL TRV for marine mammals (or surrogate carnivorous mammal). TRVs identified through literature review. |
| BERA: Realistic estimate of daily ingested dose of contaminant within DU compared with LOAEL TRV for marine mammals (or surrogate carnivorous mammal). | |||
21.3.3.4 STEP 1B, TASK 4; IDENTIFY COMPLETE EXPOSURE PATHWAYS AND POTENTIAL ROUTES OF EXPOSURE
Complete exposure pathways consist of contaminants, receptors, and routes (such as direct contact, sediment ingestion, and food chain transfer).
- Receptors: Living organisms present or potentially present at the site are the focus of the SLERA
- Exposure Medium: This part of the TGM addresses sediment as the primary exposure medium. Organisms in direct contact with the sediment may take up chemicals in their tissues and become sources of contaminants to animals that consume them. Exposure to contaminated food items (and ingested sediment) is evaluated using food chain models (see Subsection 21.3.4: Step 2, Task 3 below).
- Depth of Sediment Exposure: Benthic invertebrates typically live either on the surface of the sediment or within the top layer where water and oxygen exchange occur (the biotic zone). The default assumption of exposure depth for a SLERA is that benthic and epibenthic receptors are exposed to the top 10 cm of sediment. However, if receptors are known to burrow deeper in the sediment at a particular site, the exposure pathway to deeper sediment layers should be evaluated in the SLERA.
- Routes of Exposure: The SLERA should focus on routes of exposure most likely to be significant. Receptors living on or in the sediment are exposed primarily through direct contact; they may also be exposed to ingested sediment. Other receptors are indirectly exposed to sediment by consuming organisms that were in direct contact with the sediment.
The preliminary CSM for a SLERA relies on the published literature to predict occurrence of receptors and the trophic relationships among receptors at the site. Reports and publications written for purposes other than contaminant studies can be good sources of information on ecological processes and relationships in a given habitat type or location. For example, NOAA prepared a diagram of trophic linkages on the kaloko reef system for a report on energy flow on the Kona coastline (NOAA 2018b) (Figure 21-1). Although the NOAA project was not focused on contaminants, it provides valuable information on species occurrence and trophic relationships that could be incorporated into a SLERA in that location.
21.3.3.5 STEP 1B, TASK 5; DEVELOP THE SCREENING LEVEL PRELIMINARY CONCEPTUAL SITE MODEL
The CSM presents a description of predicted relationships between receptors and chemicals. It is an integrated model of contaminant sources, transport pathways, and receptors that represents potential contaminant dynamics at the site. CSMs range from simple diagrams to detailed illustrations of habitat emphasizing trophic transfer. To the extent possible, include expected effects of climate change, such as sea level rise, in the CSM.
Elements of a CSM
Regardless of the style, the CSM should depict how contaminants are believed to move across the site (fate and transport) and how receptors might be exposed to contaminants in various media (exposure pathways). The CSM should also identify assessment endpoints, which are the particular functional features of the ecological community to be protected, or representative surrogate species. Table 21-6 presents a list of required elements of the CSM.
| Table 21-6. Elements of a Marine Sediment Ecological CSM |
| Sources of Chemical in Marine Sediments |
|
| Contaminant Transport Pathways |
|
| Exposure Pathways to Ecological Receptors |
|
| Ecological Receptors |
|
| Modified from (USEPA 2005f): Contaminated Sediment Remediation Guidance for Hazardous Waste Sites |
The preliminary CSM developed during the SLERA may include multiple chemicals and receptors to ensure that all potentially complete exposure pathways are included. The CSM is typically updated as more information is learned about the site. For example, if the risk assessor learns that a predicted pathway is incomplete because an expected receptor does not occur at the site, then the CSM is revised to eliminate that pathway and receptor.
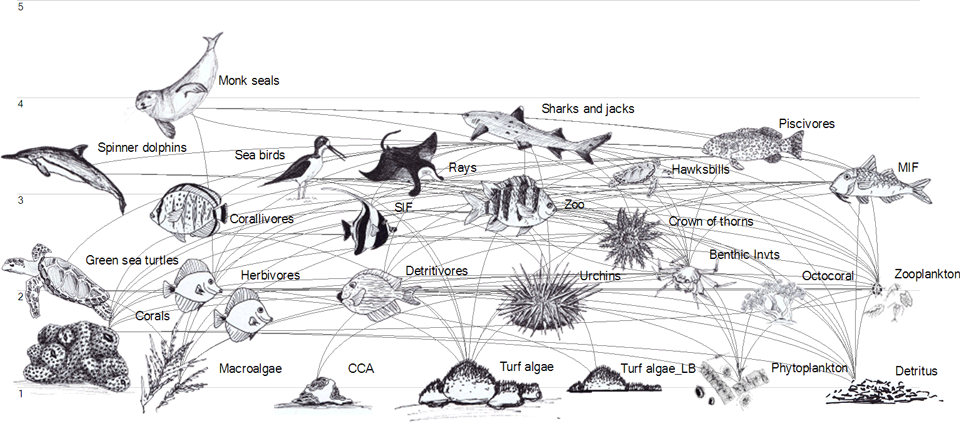
Figure 21-1. Food Chain Models Can Support Development of Conceptual Site Model
Graphical representation of the trophic linkages (i.e., who-eats-whom) within the Kaloko reef ecosystem. Each animal group within the system is identified here by an illustration (© M. Bailey); where relevant, an image of a species representative of its group is depicted. Images are not drawn to scale or proportional to the group’s biomass. The light grey horizontal lines and associated numbers represent trophic levels (position in the food web); lines connecting individual groups represent trophic links. (NOAA 2018b)
Example CSMs
The HEER Office has prepared several examples to illustrate acceptable preliminary CSMs for a marine sediment SLERA. The risk assessor may adapt one of these CSMs or develop a new CSM incorporating the required elements from Table 21-6.
- Figures 21-2 and 21-3 present two types of CSM for the same site, a rocky intertidal site such as ʻĪlio Point on Molokaʻi. Figure 21-2 is a simple diagram and Figure 21-3 is a pictorial representation.
- Figure 21-4 is a CSM for a soft-bottom bay/harbor habitat (such as Hanalei Bay, Kauaʻi or Pearl Harbor) that illustrates both direct exposure to sediment and secondary exposure to contaminated prey. This CSM would be suitable to represent bioaccumulating COPECs (such as PCBs or organochlorine pesticides) that were originally released to soil, then washed into the marine habitat. In this scenario, ingestion of COPECs associated with sediment particles is considered the principal exposure pathway.
- Figure 21-5 is a CSM prepared for a BERA at Pearl Harbor. Note the multiple sources of COPECs that contribute to the existing load in the sediment.
- Figure 21-6 presents a focused CSM that illustrates the exposure of a single receptor group (water birds) to a single COPEC (arsenic) in sediments and surface water in Waiākea Pond on Hawaiʻi Island.
- Figure 21-7 is a CSM focused on a particular class of COPECs (energetic compounds associated with discarded munitions).
Other Features to Consider in CSMs
The following considerations should be taken into account when developing CSMs for marine sediment sites in Hawaiʻi:
- At intertidal sites, the CSM must capture both high tide and low tide exposure pathways. The intertidal habitat depicted in Figure 21-3 shows the inundated state, during which large pelagic fishes and sea turtles are present. At low tide, the large organisms move off shore and seabirds become the dominant predators. The CSM must account for exposure pathways under the full tidal cycle. See (Harborne 2013) for a discussion of foraging shifts between low and high tides on reef flats.
- At sites with stream discharge or other terrestrial inputs, the CSM must reflect the seasonal flux of contaminants entering the site. For example, in Hilo Bay, Hawaiʻi, the dominant exposure pathway to marine receptors varied throughout the year. Streams discharged heavy loads of soil/sediment as suspended particulate matter during the rainy season. Contaminants associated with terrestrial sources were transported to the bay along with the fresh water. Exposure of organisms in the bay to terrestrially-derived contaminants fluctuated from station to station, influenced by proximity to stream discharge and the time interval since the last major storm (Atwood et al. 2012). The CSM at a site with substantial terrestrial input must reflect this type of variability.
- At an anchialine pond site, the CSM must be developed specifically to reflect the relatively simple but unusual food web typical of this habitat. Apart from, or in addition to, effects mediated by contamination, any physical or biological perturbation of the food web can upset the balance of species in the pond, many of which are rare, endemic, or endangered. For background on anchialine ponds (see Dalton et al. 2013).
- The wave energy at a site must be considered in the CSM because waves are influential in sediment transport, deposition, and particle sorting processes that affect exposure of organisms to contaminants. Also, some receptors thrive in high energy environments while others prefer calmer environments. Many COPECs become bound to fine-grained sediment in the field, which tend to accumulate in areas where wave energy is dissipated by vegetation, such as seagrasses and mangroves, or around coastal protrusions such as jetties and piers. When fine-grained sediments are disturbed, either naturally by storms and erosion or purposefully by dredging or construction, metals can become remobilized from the sediments into the water column (Batley et al. 2013). Organic COPECs can become more bioavailable as fine sediment particles are suspended and ingested by receptors. The U.S. Geological Survey (USGS) has conducted numerous studies of natural processes that affect erosion and deposition in Hawaiʻi. Geophysical processes affect not only where sediments accumulate, but also how receptors are exposed to contaminated sediments. To assist risk assessors in describing the wave environment at a contaminated sediment site, the HEER Office has compiled a database of geophysical information provided in USGS reports, as well as in the primary literature, including descriptions and locations of high and low energy aquatic environments; erosional and depositional areas; and other features. The risk assessor should ensure that the influence of wave action is accurately represented in the CSM.
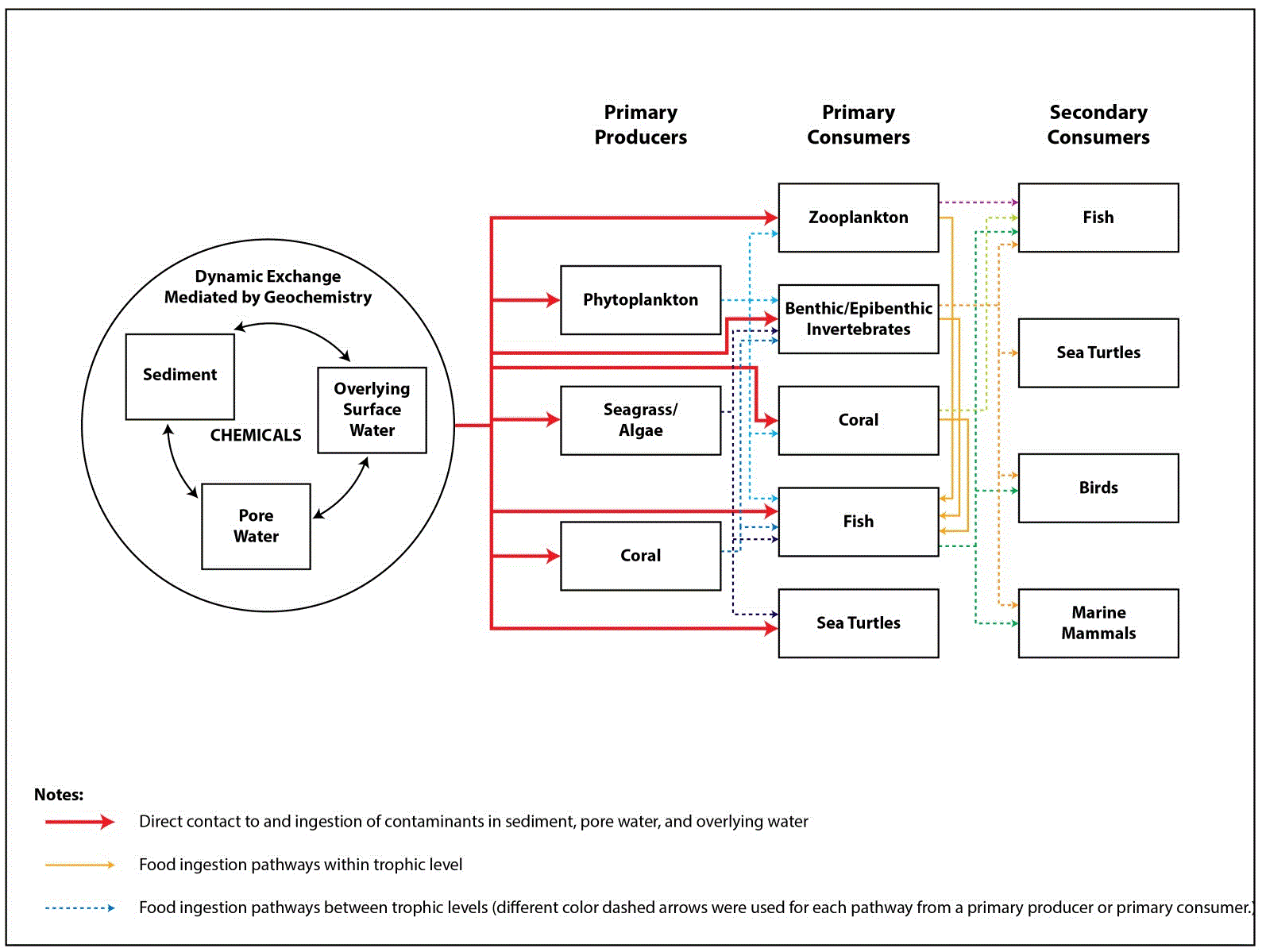
Figure 21-2. A Simple Diagrammatic Conceptual Site Model for a Rocky Intertidal Habitat with Hardbottom (such as ʻĪlio Point, Molokaʻi)
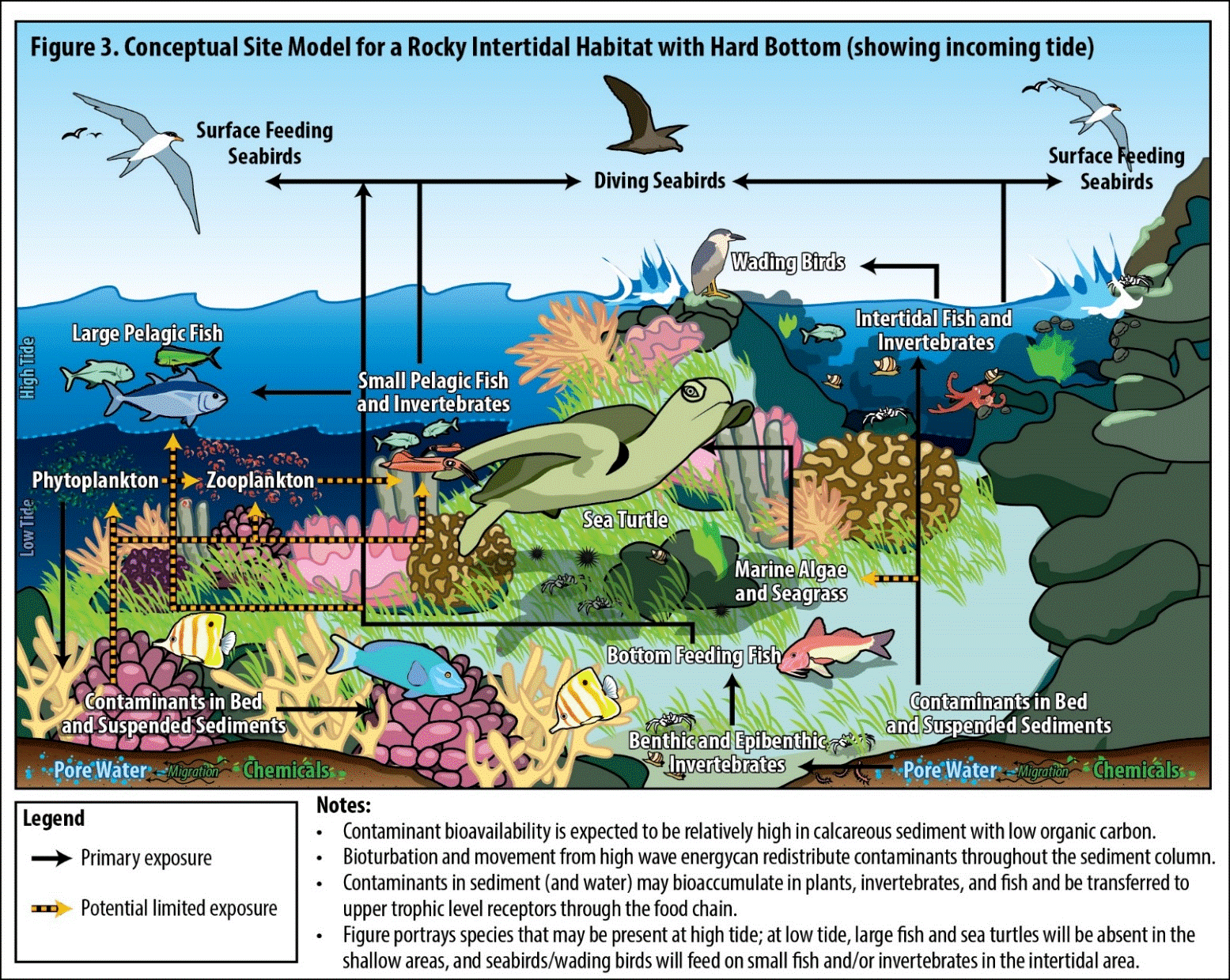
Figure 21-3. Conceptual Site Model for a Rocky Intertidal Habitat with Hardbottom
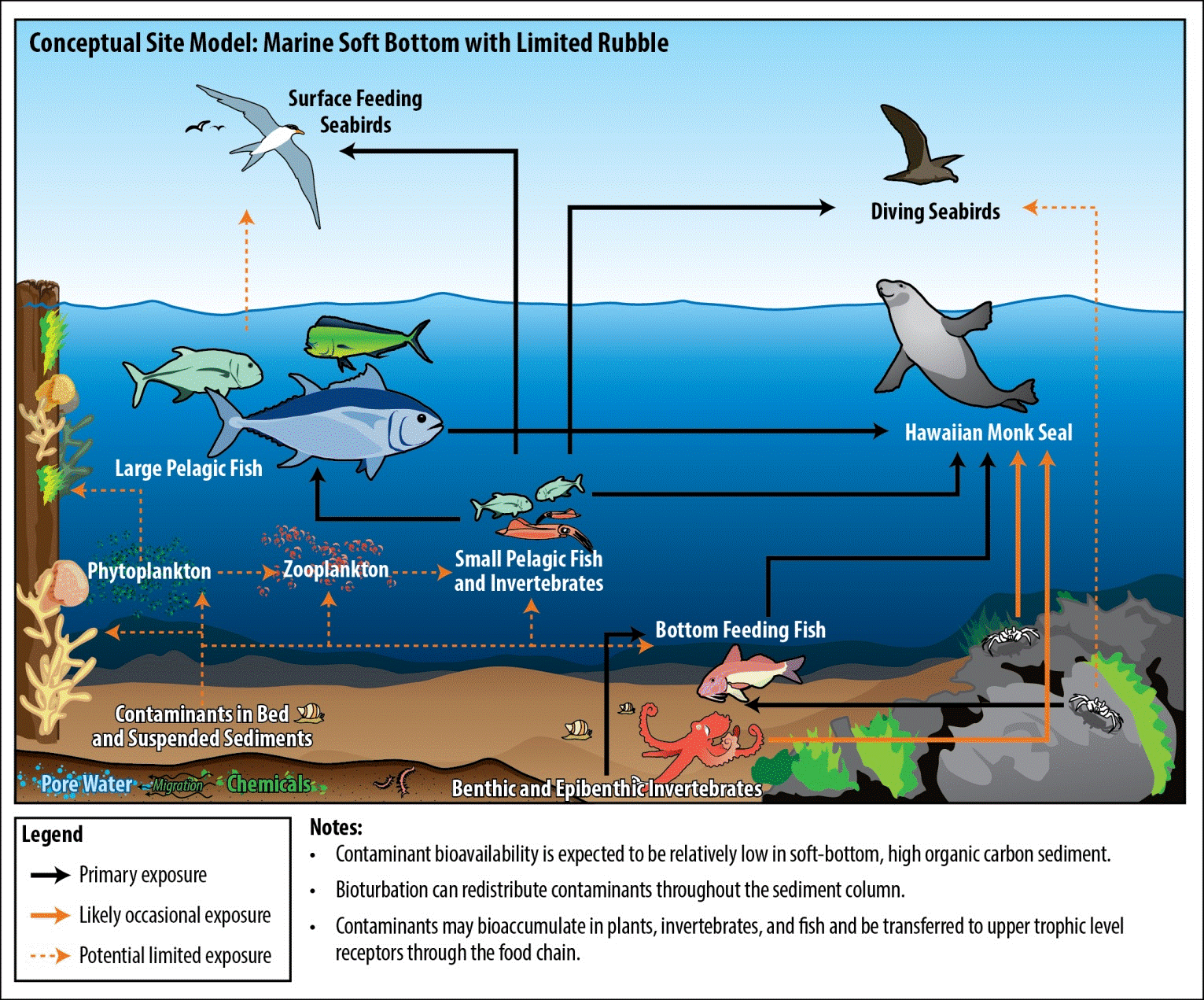
Figure 21-4. Conceptual Site Model for a Soft-Bottom Bay/Harbor Habitat (such as Hanalei Bay, Kauaʻi, or Pearl Harbor, Oʻahu)
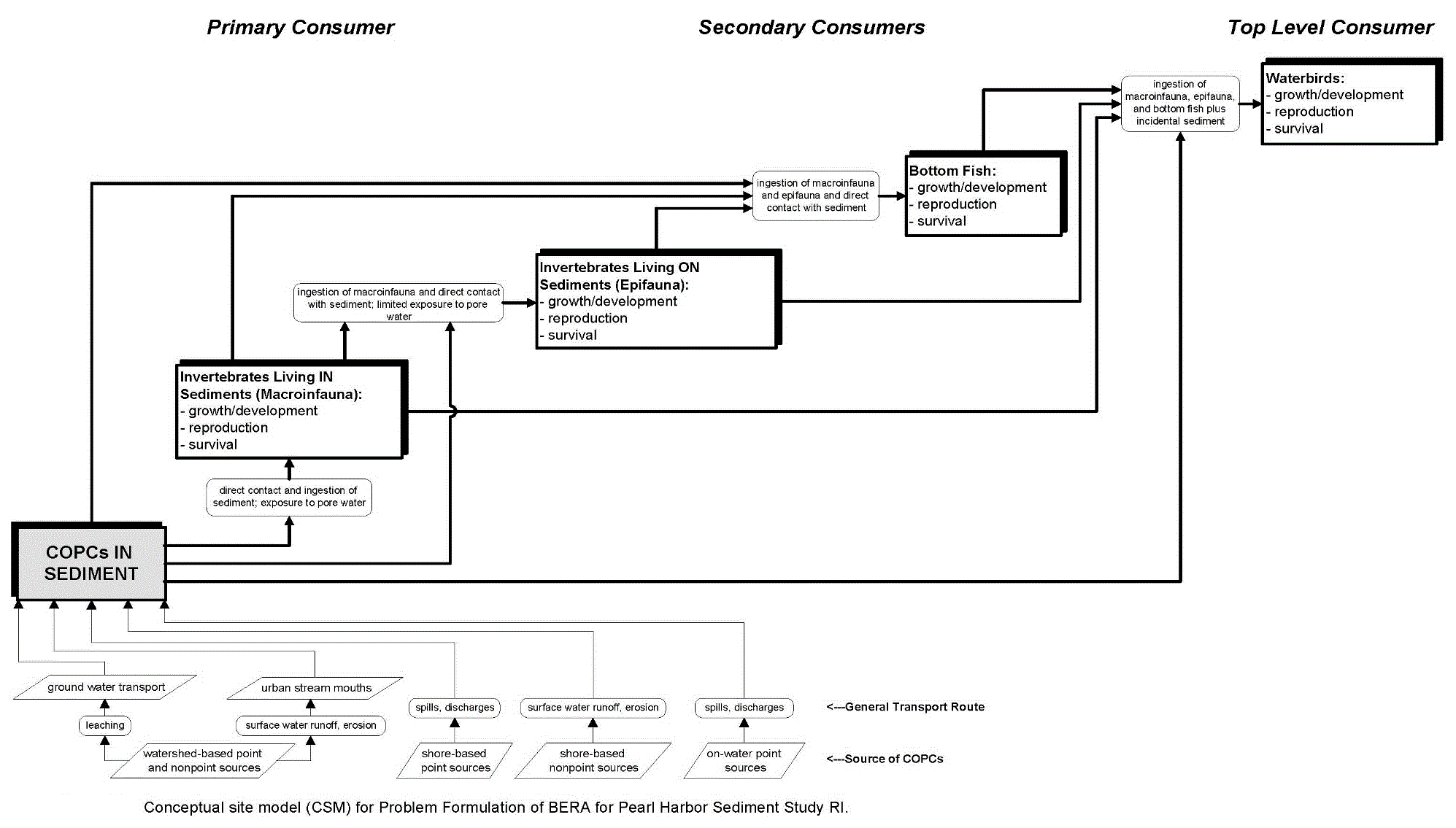
Figure 21-5. Conceptual Site Model Prepared for a BERA at Pearl Harbor
Source: (NAVFAC 2007), Figure 2-7
(Note the multiple sources of COPECs that contribute to the existing load in the sediment.)
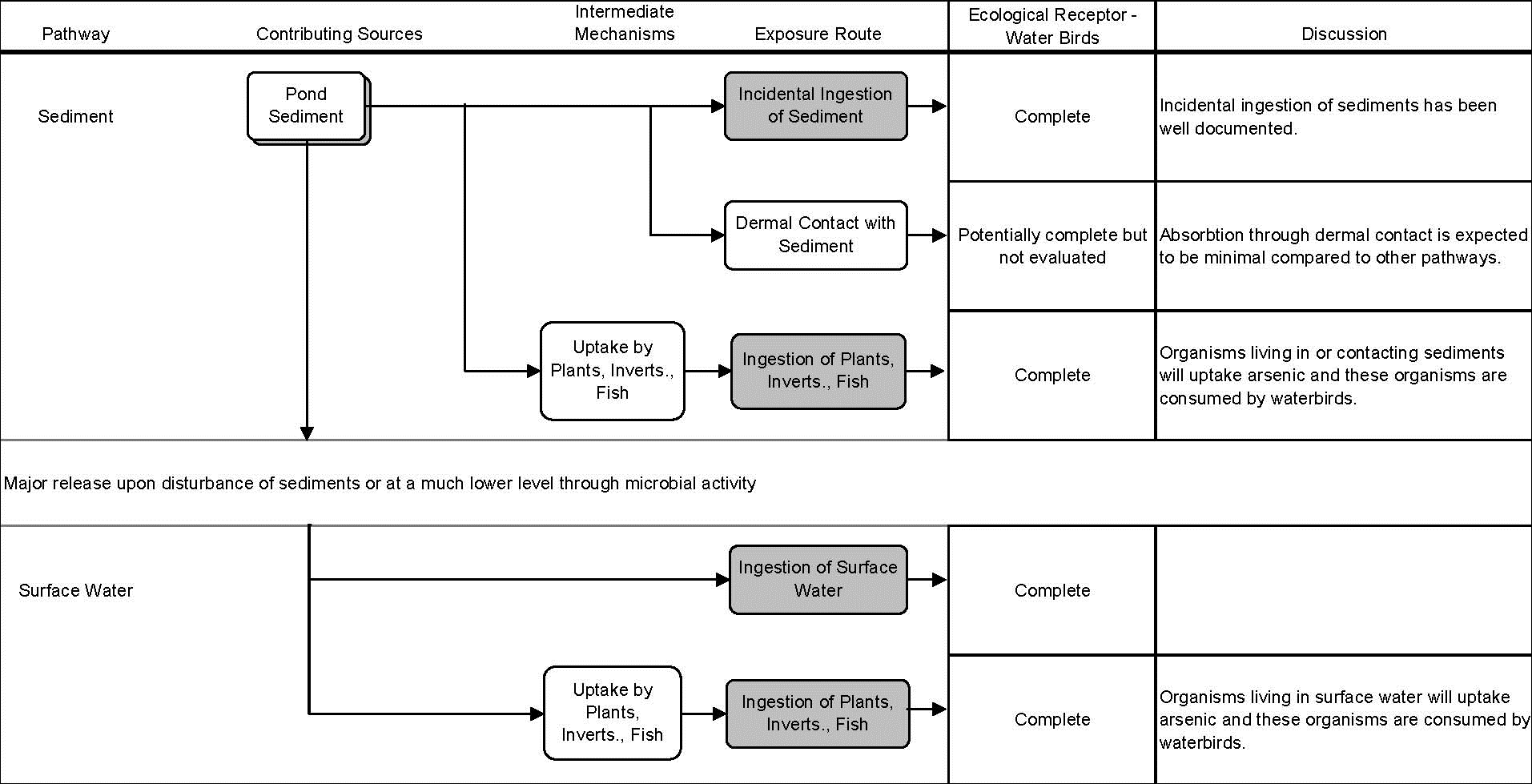
Figure 21-6. Conceptual Site Model Focused on Exposure of a Single Receptor Group (Water Birds) to a Single COPEC (Arsenic) in Sediments and Surface Water at Waiākea Pond on Hawaiʻi Island
Source: (HDOH 2005c), Figure 2-1
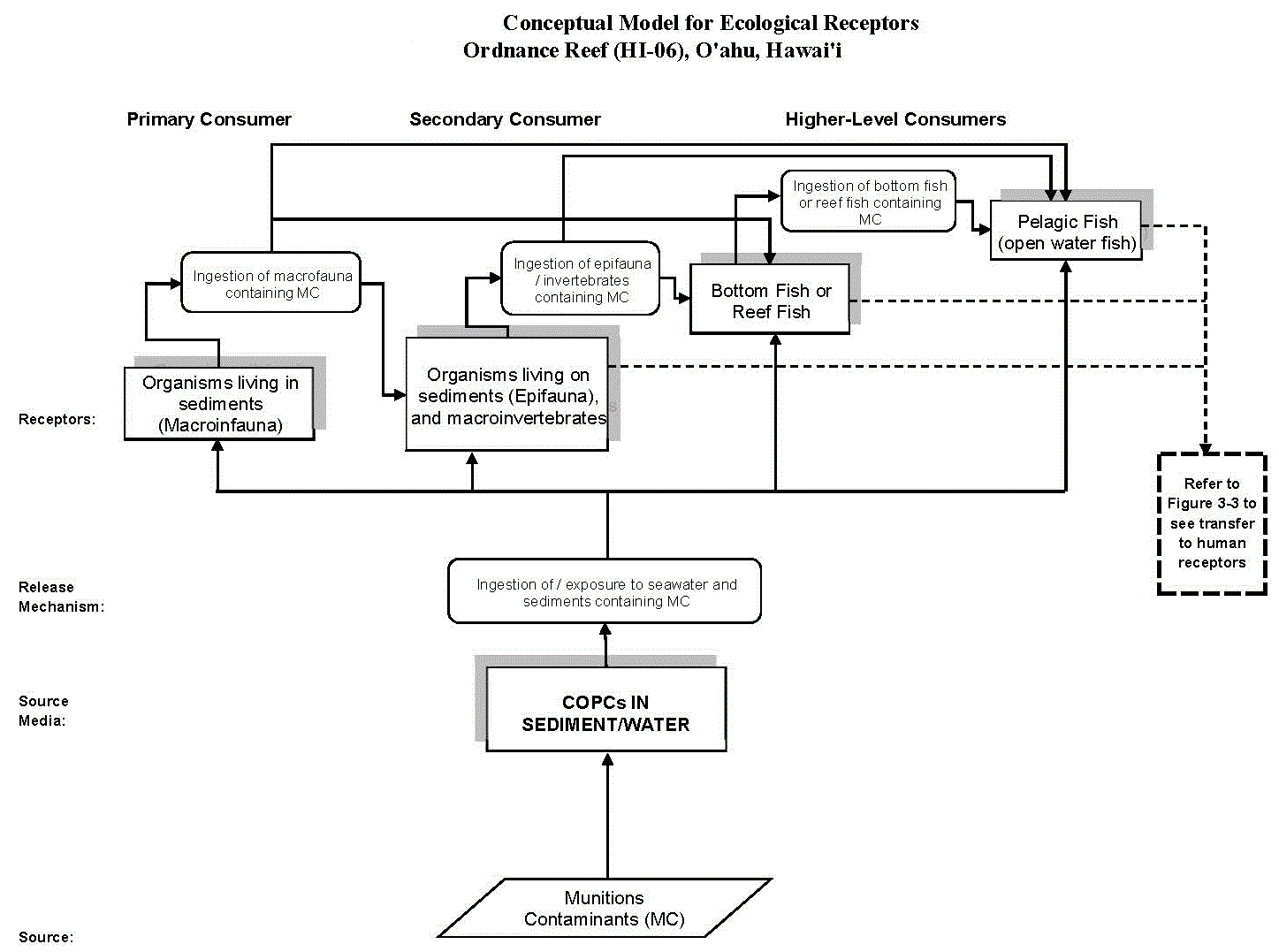
Figure 21-7. Conceptual Site Model Focused on a Single Class of COPECs (Energetic Compounds Associated with Discarded Munitions)
Source: (USACE 2012) Figure 3-2
21.3.4 STEP 2: ESTIMATING EXPOSURE AND EFFECTS
In Step 2, available site-specific data are used to estimate conservative contaminant concentrations, which are then compared with screening levels to identify (1) chemicals that may pose potential risk and (2) chemicals that may be eliminated from further investigation.
21.3.4.1 STEP 2, TASK 1; COMPILE SCREENING LEVELS
SQGs and other screening levels are compiled as part of the ERA Scoping Checklist following the examples in Tables 21-B-1 through 21B-4. If additional analytical data or screening levels have become available, update the table. The HEER Office has developed screening levels for common COPECs at sediment sites in Hawaiʻi. Each of the screening levels is used to evaluate a different aspect of potential risk to receptors, as described below.
- Sediment quality guidelines (SQG) are used to evaluate risks to receptors in direct contact with the sediment, especially benthic invertebrates. The SQGs were derived from large datasets on toxicity to benthic invertebrates under a variety of field conditions. Although the SQGs are not necessarily protective of seagrasses, marine algae, fish, or receptors that are not intimately exposed to sediment, they serve as surrogates during the SLERA. The HEER Office will add SQGs to this guidance as they become available. See Table 21-7 and see Appendix 21-E.
- HEER Office Environmental Action Levels (EALs) used to evaluate aquatic toxicity (aquatic habitat goals), surface water, and groundwater are available for screening of chemicals in water (see EAL Surfer). See detailed Tables in the EAL Surfer tool for listings of aquatic toxicity and surface water EALs for marine, estuarine, or freshwater environments, as applicable.
- Toxicity reference values (TRV) are daily doses of ingested chemicals used to evaluate risk to birds and mammals that are exposed to contaminants primarily through ingestion of contaminated food items (as well as sediment and water).
- Critical body residues (CBR) are used to evaluate risk to receptors from chemicals accumulated by all routes into their tissues. CBRs are available for only a few receptors at this time.
HEER Office Interim Sediment Quality Guidelines
HEER Office SQGs are used to evaluate the potential for sediments to pose a risk to benthic invertebrates through direct exposure. The concentration below which sediments are considered safe for benthic marine organisms is called the interim “No Effect SQG.” The concentration above which adverse effects are indicated on benthic marine organisms may occur is called the interim “Potential Effect SQG.” Chemicals known or expected to bioaccumulate are indicated on Table 21-7 and may require additional evaluation, as described in Appendix 21-E.
The SQGs are considered interim because they are subject to revision as new data become available. The HEER Office anticipates that the HDOH interim SQGs will be revised as warranted by a review of new toxicity data reported from other tropical marine ecosystems, including the ANZEC/ARMCANZ ecotoxicology group. In the future, a range of revised SQGs will represent sediments that vary in percent organic carbon and grain size.
The HEER Office interim SQGs incorporate the Effects-Range Low (ER-L) and Effects-Range Median (ER-M) sediment levels published by (Long and Morgan 1990) and modified by (Long et al. 1995), as well as the ANZECC/ARMCANZ interim SQGs derived from other sources. Interim SQGs for 2,3,7,8-TCDD, which were not available from ANZECC/ARMCANZ or NOAA, were adopted from Canadian Council of Ministers of the Environment (CCME 2001).
The HEER Office considers the chemicals listed in Table 21-7 the most likely to be potential risk drivers at marine sediment sites in Hawaiʻi. Chemicals detected in sediment for which no HEER Office interim SQG is available should be screened using the most recent publicly available literature available. Suggested sources are listed below:
- SQGs from (Simpson et al. 2013) and related documents
- Marine sediment screening levels from sources presented in the U.S. Department of Energy, Risk Assessment Information System – Ecological Benchmark Tool (USDOE 2018).
- Marine sediment screening levels from sources presented in the NOAA Screening Quick Reference Tables (Buchman 2008)
| Table 21-7. HDOH HEER Office Interim Sediment Quality Guidelines for Selected Chemicals | ||
| Analyte | Recommended Interim Sediment Quality Guidelines for Direct Exposure | |
| No Effect SQG | Potential Effect SQG | |
| Inorganic Chemicals (mg/kg dry weight) | ||
| Arsenic | 20 | 70 |
| Copper | 34a | 270 |
| Lead | 50 | 220 |
| Mercury | 0.15 | 1 |
| Tributyltin (µg/kg Sn/kg dry weight) | 5 | 70 |
| Zinc | 200 | 410 |
| Organic Compounds | ||
| Pesticides/PCBs/Dioxins (µg/kg dry weight) | ||
| 4,4′-DDD | 2 | 20 |
| 4,4′-DDE | 2.2 | 27 |
| Total DDTs | 1.6 | 46 |
| Total Chlordane | 0.5 | 6 |
| Dieldrin | 0.02 | 8 |
| Endrin | 0.02 | 8 |
| Total PCBs | 23 | 180 |
| TEQ Dioxins and Furans | 0.00085 | 0.0215 |
| Semivolatile Organic Compounds (µg/kg dry weight) | ||
| Acenaphthene | 16 | 500 |
| Acenaphthylene | 44 | 640 |
| Anthracene | 85 | 1100 |
| Benzo(a)anthracene | 261 | 1600 |
| Benzo(a)pyrene | 430 | 1600 |
| Chrysene | 384 | 2800 |
| Dibenzo(a,h)anthracene | 63 | 260 |
| Fluoranthene | 600 | 5100 |
| Fluorene | 19 | 540 |
| Naphthalene | 160 | 2100 |
| Phenanthrene | 240 | 1500 |
| Pyrene | 665 | 2600 |
| Sum HMW PAHs | 1700 | 9600 |
| Sum LMW PAHs | 552 | 3160 |
| Total PAHs | 4000 | 45000 |
| HMW LMW µg/kg mg/kg PAH PCB SQG TEQ |
High molecular weight Low molecular weight Microgram per kilogram Milligram per kilogram Polycyclic aromatic hydrocarbon Polychlorinated biphenyl Sediment quality guideline Toxic equivalent |
|
Notes:
|
||
The chemicals on the HEER Office SQG table (Table 21-7) are also known as common bioaccumulating chemicals, based on a review of technical manuals prepared by USEPA, other states, and international organizations. Therefore, these chemicals should also be considered potential bioaccumulators, and evaluated accordingly using food chain models (see Step 1b, Task 3). The risk assessor should also consider other technical sources of information when determining whether chemicals detected in sediment at a site may be bioaccumulators. The Bioaccumulation Testing and Interpretation for the Purpose of Sediment Quality Assessment, Status, and Needs (USEPA 2000i) provides technical direction on identifying bioaccumulators. More detailed guidance on evaluating risk of bioaccumulating chemicals is in Appendix 21-E.
Toxicity Reference Values
A TRV is an ingested daily dose of a chemical associated with a designated effect level. A low TRV is a conservative value consistent with a chronic no observable adverse effect level (NOAEL). A high TRV is consistent with a lowest observable adverse effect level (LOAEL). When compared to site-specific doses ingested by receptors, the high TRV should be used to identify sites posing potential risk to birds or mammals. Conversely, the low TRV is a dose level below which no adverse effects are expected.
The HEER Office has not compiled a comprehensive list of TRVs for all receptors. The risk assessor may select TRVs based on site-specific receptors and exposure conditions and provide technical rationale for the TRVs selected. TRVs are available from several sources in the literature, including, but not limited, to the following:
- TRVs developed by the U.S. Navy for 20 chemicals common at San Francisco Bay area naval installations, including 12 metals and metalloids (arsenic, butyltins, cadmium, cobalt, copper, mercury, lead, manganese, nickel, selenium, thallium, and zinc), five pesticides (aldrin, DDT, heptachlor, lindane, and methoxychlor) and three other organic compounds (benzo(a)pyrene, naphthalene, and total polychlorinated biphenyls) (Navy 1998). Several of the Navy TRVs have been updated using more recent toxicological studies (CalDTSC 2009)
- Toxicological Benchmarks for Wildlife (Sample et al. 1996)
- FCSAP Supplemental Guidance for Ecological Risk Assessment Selection or Development of Site-specific Toxicity Reference Values (Azimuth 2010). This document does not present specific TRVs but list several sources of TRVs.
- Recommendations for the Development and Application of Wildlife Toxicity Reference Values (Allard et al. 2010). This document does not present specific TRVs but presents recommendations on the derivation and application of wildlife TRVs.
- EPA Ecological Soil Screening Level Documents (USEPA 2005g) and supporting documents). Although these documents pertain to soil, some of the toxicological literature cited within them is relevant to birds and mammals exposed to chemicals in surface water and sediment.
- Los Alamos National Laboratory, ECORISK Database (Release 4.1) (LANL 2017). This database presents TRVs for several chemicals and receptors.
Note that TRVs used in ERAs in Hawaiʻi are provided in the species profiles, where available (See Appendix 21-A). The HEER Office does not necessarily endorse the use of the particular TRVs presented in earlier ERAs but does recommend that the risk assessor make use of existing literature to select and provide rationale for TRVs suitable to the site.
Critical Body Residues
The CBR can be used to evaluate risk to a receptor based on a chemical concentration in its tissue. However, CBR data are available for only a few chemicals and selected species from a limited number of locations. Few, if any, of the published CBRs cited are for native Hawaiian species. No standard CBR values have been developed by EPA or other national agencies. Limited CBR data are available from the following sources:
- Linkage of Effects to Tissue Residues: Development of a Comprehensive Database for Aquatic Organisms Exposed to Inorganic and Organic Chemicals (Jarvinen and Ankley 1999). Most of the available data are for freshwater species, although some marine and estuarine species are included.
- Guidance for Assessing Bioaccumulative Chemicals of Concern in Sediment provides freshwater and marine CBRs for metals, pesticides, PCBs, and 2,3,7,8-TCDD TEQs (ODEQ 2017).
- Environmental Residue Effects Database (ERED) is a searchable compendium of CBRs derived by USEPA and the USACE from literature published in the 1960s to 1990s. (US Army 2018)
- Dredged Material Evaluation and Disposal Procedures User Manual (DMMP) (USACE 2016) lists target tissue concentrations for several chemicals.
- Environmental Contaminants in Biota: Interpreting Tissue Concentrations, Second Edition (Beyer and Meador 2011) summarizes data on CBR for numerous species and contaminants.
21.3.4.2 STEP 2, TASK 2; CALCULATING CONTAMINANT CONCENTRATION(S) IN SEDIMENT AND WATER
At a minimum, the SLERA requires site-specific sediment concentrations. The preferred approach to estimating exposure concentrations at a sediment site is to use MIS sampling to represent the typical exposure of receptors within a DU. The general guidance in the TGM on developing a sampling plan for a sediment investigation is applicable to an ERA (see TGM Sections 3, 4, and 5). However, the designation of DUs is more complex for an ERA because no single DU is appropriate for all ecological receptors at a site (See Appendix 21-C).
Stationary and relatively immobile species such as algae, benthic infauna, and coral are primarily exposed to chemicals in sediment through direct contact. The MIS concentration detected in a DU is used as the representative contaminant concentration in the SLERA. Assuming laboratory detection limits are lower than the SQGs, non-detects are treated as zero values. If the laboratory detection limit exceeds the SQG, the detection limit is used as the reported value for all nondetects. (In this case, the data should be scrutinized and laboratory methods reviewed so that detection limits appropriate for a SLERA can be achieved.)
If site-specific concentrations are available for surface water, sediment pore water, or groundwater discharging to the site, the MIS detected concentration is used as the contaminant concentration for the SLERA (given the protocol for estimating nondetects in the previous paragraph). Samples should be analyzed for dissolved concentrations for constituents that have WQC based on dissolved concentrations.
The SLERA is purposefully designed to be conservative, evaluating the worst-case exposure scenario and often overestimating contaminant concentrations in early steps. Subsequent steps allow refinement of conservative assumptions to reflect site-specific conditions that may reduce estimated contaminant levels or risk.
21.3.4.3 STEP 2, TASK 3; ESTIMATING DAILY INGESTED DOSE TO BIRDS AND MAMMALS
The SQG are considered protective of algae, benthic invertebrates, and fish exposed directly to sediment but cannot be used to evaluate risk to birds or mammals feeding on prey at a contaminated sediment site. Risk to birds and mammals ingesting sediment, water, and prey at a site is evaluated using food chain modeling to estimate the dose of a chemical ingested by these animals.
Tissue concentrations are a key component of dose estimates to birds and mammals, but are not always available during a SLERA. If tissue concentrations from organisms collected at the site or from organisms exposed to site-sediment in the laboratory are available, site-specific doses to birds and mammals can be estimated. Site-specific tissue concentrations (also known as CBRs) can also be used to estimate direct effects to the organisms from contaminant body burdens. If no tissue data are available, chemical concentrations in tissue may be estimated using concentrations in sediment, literature BSAFs, and parameter assumptions (see Appendix 21-F).
Ingested doses of bioaccumulative chemicals are estimated using food chain models. The dose estimate represents the mass of chemical ingested per day, indexed to the receptor’s body weight (mg/kg-body weight/day). Daily ingested doses are estimated for higher trophic level receptors (birds and mammals) that are exposed to contaminants primarily through their diet rather than through direct contact with sediment. Where appropriate, the dose estimate should include incidental sediment ingestion. For example, the Hawaiian monk seal is reported to consume substantial amount of sediment when it hauls out on beaches. The risk assessor should review the relevant literature on key receptors at the site to determine the need to include sediment ingestion in the dose for a given receptor.
The ingested dose should be estimated using the following generic exposure equation. The equation can be modified, as necessary, based on the specific exposure pathways evaluated in the SLERA:
Where:
- ED = exposure dose (mg/kg-day)
- Cf = chemical concentration in food (mg/kg)
- Cs = chemical concentration in sediment (mg/kg)
- If = food ingestion rate (kg/day)
- Is = incidental sediment ingestion rate (kg/day)
- SUF = site use factor (site/species home range – cannot exceed 1.0) (unitless)
- BW = body weight (kg)
Chemical concentrations and ingestion rates (for sediment and food) should be reported in dry weight. If tissue concentrations are reported by the analytical laboratory in wet weight, dry weight concentrations can be estimated using either laboratory measures or standard default values for percent moisture.
For the SLERA, the estimated daily dose is intentionally biased high so that any error will be toward indicating greater risk than is present. In later phases of the ERA, biases are relaxed in favor of more realistic assumptions. For example, the estimated dose in the SLERA should be based on the
- Maximum chemical concentration in sediment and food;
- Maximum ingestion rates for sediment and food;
- Lowest body weight;
- Highest site use factor; and
- Most sensitive life stage present at the site.
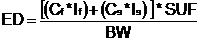
- Where:
- ED = exposure dose (mg/kg-day)
- Cf = chemical concentration in food (mg/kg)
- Cs = chemical concentration in sediment (mg/kg)
- If = food ingestion rate (kg/day)
- Is = incidental sediment ingestion rate (kg/day)
- SUF = site use factor (site/species home range – cannot exceed 1.0) (unitless)
- BW = body weight (kg
The HEER Office provides species profiles for selected receptors at coastal marine sediment sites (Table 21-8). Species profiles are in Appendix 21-A. Values for exposure parameters required in the food chain model, such as body weight and home range, are included in the species profiles when available. The risk assessor should review the current published literature to obtain additional information where data are not provided.
| Table 21-8. Selected Species Profiles | |
| Receptor Group | Selected Species* |
| Marine Algae | Sea lettuce (Ulva fasciata) |
| Invertebrates | Samoan crab (Scylla serrata) |
| Kona crab (Ranina ranina) | |
| White crab (Portunus sanguinolentus) | |
| Helmet urchin (Colobocentrotus atratus) | |
| Hawaiian limpet (Cellana exarata) | |
| Day octopus (Octopus cyanea) | |
| Polychaete (Neanthes arenaceodentata) | |
| Lobe coral (Porites lobata) | |
| Black sea cucumber (Holothuria atra) | |
| Fish | Goatfish (Mulloides vanicolensis) |
| Hawaiian flagtail (Kuhlia sandvicensis) | |
| Convict tang (Acanthurus triostegus) | |
| Pacific sergeant (Abudefduf abdominalis) | |
| Mozambique tilapia (Oreochromis mossambicus) | |
| Spectacled parrotfish (Chlorurus perspicillatus) | |
| Yellowbar parrotfish (Calotomus zonarchus) | |
| Moray eel (Muraenidae) | |
| Birds | Wedge-tailed shearwater (Puffinus pacificus) |
| Black-crowned night heron (Nycticorax nycticorax hoactli) | |
| Hawaiian coot (Fulica alai) | |
| Sea Turtles | Green sea turtle (Chelonia mydas) |
| Marine Mammals | Monk seal (Monachus schauinslandi) |
| * See Appendix 21-A for profiles of these species. | |
Calculate Critical Body Residues
The HEER Office does not require that tissue concentrations be obtained during the SLERA. However, tissue samples collected to support a human consumption study or other phase of investigation at the site may be available for inclusion in the SLERA. The risk assessor should present the available tissue data in tabular form with details on the sample date, location, species, size of specimen, body part, analytical methods, and results (with data qualifiers). If the tissue samples are composites of more than one individual organism, the details above should be provided for all individuals in the composite. (When possible, tissue concentrations should be measured in single individuals rather than composites for comparison to CBRs.) The maximum detected tissue concentration is used as the exposure concentration in the SLERA. Non-detects are treated as zero values when detection limits are acceptable (see Step 2, Task 2).
21.3.4.4 STEP 2, TASK 4; CALCULATE SITE-SPECIFIC HAZARD QUOTIENTS
Risk calculations in the SLERA are simple and straightforward for chemicals that are not considered bioaccumulators. The maximum exposure concentration is divided by the no-effect screening level to calculate a hazard quotient (HQ). If the resulting HQ is greater than 1.0, that chemical is designated a chemical of potential ecological concern (COPEC) and should be evaluated further. If the HQ is less than 1.0 for that chemical, it is eliminated as a COPC and dropped from further consideration. Chemicals without screening levels are retained as COPECs at this point in the process. To compensate for the uncertainty inherent in single chemical SQGs, the initial step of the SLERA is purposefully biased toward including chemicals that may not pose a risk rather than eliminating COPECs that may pose a risk, by use of conservative exposure assumptions. This bias toward including COPECs is corrected during later phases of the ERA (i.e., Step 3a or the BERA) in which the COPEC list is refined using more realistic assumptions and site-specific exposure data. The HQs for receptors directly exposed to sediment should be calculated as follows:
HQsediment = maximum sediment concentration/no effect SQG
Risks from chemicals that bioaccumulate can be evaluated using the equation above to assess direct toxicity to organisms. If the resulting HQ is less than 1.0, no direct toxicity is indicated. However, a bioaccumulating chemical cannot be eliminated as a COPEC based on a simple sediment screen because it may be bioaccumulated even when its concentration in sediment is less than the SQG. Risk posed by food chain transfer of contaminants is evaluated using TRVs derived for higher trophic level receptors. The estimated daily dose of a chemical in a given receptor is compared with the no-effect TRV to calculate an HQ:
HQ-TRVlow = estimated daily dose/no-effect TRV
Bioaccumulating chemicals can also pose a direct risk to the receptor in the form of causing neurological, developmental, or other impairment. The concentration of a bioaccumulating chemical in the whole body (or specific tissue type) of a receptor can be compared to the concentration demonstrated to cause an adverse effect on that receptor (or a surrogate species). When tissue effect levels for comparable species and tissue types are available in the literature, risk is estimated by comparing site specific tissue concentrations to CBRs from the literature:
HQtissue = site-specific tissue concentration/CBR
21.3.4.5 STEP 2, TASK 5; DECISION CHECKPOINT
By this stage of the process, all available sediment, water, and tissue data have been screened against no-effect screening levels and HQs have been calculated. Chemicals for which all HQs are less than 1.0 can be eliminated from further evaluation. Chemicals for which at least one HQ is greater than 1.0 are retained as COPECs. The HEER Office recommends the SLERA include a summary table supporting the decision to eliminate or retain each chemical.
21.3.5 STEP 3A: REFINE SCREENING LEVEL DEFAULT ASSUMPTIONS
The COPECs retained at the end of Step 2 were shown to pose potential risk to receptors when conservative assumptions were used. Step 3A is focused on refining the list of COPECs to represent more realistic site-specific conditions. The objective of the COPEC refinement is to identify chemicals that significantly contribute to potentially unacceptable levels of ecological risk and eliminate from further consideration those chemicals that are not likely causing a significant risk. This step consists of refining the conservative exposure assumptions/concentrations used to evaluate potential risks to ecological receptors and re-evaluating the analytical data using screening levels that are more appropriate for the assessment endpoints.
This refinement may result in eliminating chemicals as COPECs for some receptors but retaining them as COPECs for other receptors. For example, a chemical might be retained as a COPEC for benthic invertebrates but eliminated as a COPEC for shorebirds. This is important because if the site proceeds to a BERA, the studies in the BERA should focus only on the chemicals-receptor pairs for which risk is predicted. The following tasks will support a decision regarding the need for further evaluation.
21.3.5.1 STEP 3A, TASK 1; CONDUCT BACKGROUND SCREENING
The risk assessor should compare site-specific concentrations of COPECs with regionally-appropriate background, ambient, or reference concentrations to ensure that only site-related chemicals are carried through to the BERA. Inorganic chemicals pose unique difficulties for ERAs because of the role of site-specific geology in influencing exposure and effect concentrations. Background evaluations for sediment in Hawaiʻi are complicated by spatial heterogeneity of volcanic and coralline sediment types.
In the absence of CBRs for selected receptors, the risk assessor may compare site-specific tissue concentrations with results from similar habitats or regions considered to be “unimpacted” by chemicals or to represent “background” tissue concentrations. The HEER Office is compiling tissue concentrations reported as “background” or “reference” in various published literature and reports. The values are not considered to represent “no effect” concentrations because the samples were not associated with toxicity testing. At best, the “reference” or “background” tissue concentrations indicate the range of concentrations existing in the area outside of known contaminated sediment sites. The risk assessor may compare site-specific tissue concentrations with the “reference tissue” results for the same species and habitat. Such comparisons are necessarily limited by uncertainty, yet they can provide a useful context for interpreting site-specific data. The relative magnitude of site-specific tissue concentrations compared with reference concentrations may indicate the need for further tissue sampling during the BERA or may strongly suggest that chemicals are not accumulating in tissues at the site to any measurable degree. The identification and interpretation of background, ambient, or reference concentrations should be discussed with the HEER Office before proceeding with the next task.
21.3.5.2 STEP 3A, TASK 2; EVALUATE MAGNITUDE OF SCREENING LEVEL EXCEEDANCE AND FREQUENCY OF DETECTION
Although the magnitude of risks may not relate directly to the magnitude of a criterion exceedance, the magnitude of the criterion exceedance may be used in a weight-of-evidence approach to determine the need for further site evaluation. The greater the criterion exceedance, the greater the probability and concern that an unacceptable risk exists.
Likewise, the frequency of chemical detection and spatial distribution of concentrations greater than the screening levels may indicate the need for additional investigation. A chemical detected at a low frequency typically is of less concern than a chemical detected at higher frequency if toxicity and concentrations and spatial areas represented by the data are similar. All else being equal, chemicals detected frequently are given greater consideration than those detected relatively infrequently. In addition, the spatial distribution of a chemical may be evaluated to determine the area that a sample represents. The risk assessor should discuss magnitude and frequency distributions with the HEER Office to resolve any issues before continuing with the SLERA.
21.3.5.3 STEP 3A, TASK 3; REFINE CONSERVATIVE EXPOSURE ASSUMPTIONS
Initial steps in the SLERA use assumptions of 100 percent bioavailability, high site use by sensitive receptors, representative contamination concentrations, and other factors to ensure that a chemical is not excluded from the SLERA if it poses an unacceptable risk. In Step 3a, more realistic site-specific exposure values replace the default values.
- Bioavailability: When selecting chemicals as COPECs in the SLERA, it is typically assumed that the chemicals are 100 percent bioavailable. However, in the COPEC refinement, the potential bioavailability of the chemicals can be evaluated by considering total organic carbon (TOC) and grain size data. Typically, this evaluation is more qualitative than quantitative in the SLERA. However, in a BERA, bioavailability can be measured directly through uptake in living organisms. Guidance on adjusting the assumption of 100 percent bioavailability is in Appendix 21-F.
- Site Use: The conservative default value of 100 percent site use assumes that an organism spends all of its time in contact with contaminants at the site. For some mobile species, this assumption is clearly unrealistic, and a more representative site use factor may be used.
- Contaminant Concentrations: The most conservative and reasonably representative contaminant concentration for a specific target chemical is used for initial comparison to applicable screening levels, and some potential COPECs may be eliminated from the SLERA using this approach. However, smaller or additional DUs and/or more representative sampling techniques may be used during Step 3a to support further evaluation of the site.
21.3.5.4 STEP 3A, TASK 4; OBTAIN HEER OFFICE CONCURRENCE ON REFINEMENTS
Provide the HEER Office with tables, text, figures, or other defensible rationale for refining the exposure assumptions. After reviewing the submitted materials, the HEER Office may accept the refinements or request a meeting to discuss the rationale and assumptions so that consensus can be reached.
21.3.5.5 STEP 3A, TASK 5; RECALCULATE HQS USING REFINED EXPOSURE ASSUMPTIONS
Recalculate HQs using more realistic estimate of contaminant concentration and screen against background concentrations. Prepare a summary table of COPECs eliminated and retained and provide rationale for the decisions. If risk is below applicable screening levels (or approved alternative screening level) for all chemicals, the SLERA is complete and the site can move to closure. If COPECs are retained and risk is potentially unacceptable, the site will continue to the BERA (Subsection 21.6).
21.3.5.6 STEP 3A, TASK 6; DEVELOP SLERA RISK CHARACTERIZATION AND DECISION
Risk characterization in the SLERA focuses on the summary of HQs prepared in Step 3A, Task 5 and a discussion of uncertainty and data gaps to be addressed in the BERA.
21.3.6 UNCERTAINTY
During the risk characterization phase, the exposure and effects data are interpreted within the context of other site-specific information. Specifically, various sources of uncertainty are evaluated so that the risk assessor can provide a realistic description of risks posed by contaminants at the site. Uncertainty stems from many sources, including the extrapolation of exposure and effects data form one species to another. Efforts to customize the ERA to tropical marine conditions and native Hawaiian species will greatly reduce this source of uncertainty and strengthen the risk characterization. Conversely, modifying existing toxicity tests and adapting protocols to accommodate the environmental conditions that prevail in Hawaiʻi may introduce additional uncertainty in the short term. Such trade-offs are explicitly recognized and addressed in the Sediment Quality Assessment Handbook (Simpson et al. 2005). The following paragraphs present some of the key uncertainties in SLERAs, and where applicable, how the uncertainties relate to sites in Hawaiʻi.
Uncertainty in Ecotoxicity
The HEER Office recommended interim SQGs specifically acknowledge that uncertainty stems from gaps in the science of toxicology, particularly in tropical marine ecosystems. One fundamental source of uncertainty stems from the derivation of single-chemical trigger values from toxicity tests using field-collected sediments containing multiple contaminants. Attributing toxic effects to any one of the many chemicals in such sediments leads to uncertainty that must be addressed in controlled laboratory investigations using single contaminants (Batley and Simpson 2008). The ANZECC/ARMCANZ is actively working to develop bioassays using native Australian or New Zealand species that will better reflect the genetic and ambient environmental conditions in sediments there. Some opportunity exists to adapt the Australian bioassays by substituting native Hawaiian species of similar taxonomic and functional characteristics. Therefore, although toxicity testing is typically not conducted until the BERA, the use of native Hawaiian species as test organisms for toxicity tests is encouraged, when applicable, to reduce uncertainty.
Some ecological risk investigations have been conducted in tropical marine regions, but Australia has developed an organized national program to tailor EPA and ASTM International (ASTM) protocols to tropical marine ecosystems. Although the Australian program is still in a fledgling state, many of the foundational principles are congruent with Hawaiʻi’s goal to develop a state-specific ERA program. The Australian program recognizes the EPA framework and the large body of subsequent work on refining questions of metals bioavailability in whole sediments (Batley and Simpson 2008). The Australian group has focused on developing bioassays that reflect reasonable exposure and effects conditions for local habitats (see below). Finally, that group has implemented a regionalized program that incorporates land use, climate, and contaminant source data specific to a watershed so that background conditions can be properly evaluated (Australian Government 2006).
Uncertainty in Exposure
As indicated above, tissue samples can provide a direct measure of the bioavailability of chemicals. However, there is uncertainty in where and how they accumulated the chemicals (i.e., sediment, surface water, food, or a combination). Also, the choice of organisms, portion analyzed (whole body, fillet, liver, etc.), environmental parameters (i.e., pH, TOC, grain size), along with other factors that influence bioaccumulation.
Particulate metal concentrations are nearly always higher in fine-grained sediments (<63 μm) because smaller sediment particles have a higher surface area and more binding sites available for metals (Angel et al. 2012). Although, HDOH does not recommend biasing sediment collection methods to only collect fine-grained sediments, sampling techniques must be appropriate to ensure that the finer-grained fractions are not lost during sample collection. For example, ponar samplers often allow silts to escape as the sampler is being lifted. A coring device may be more appropriate for ensuring that fine-grained sediments are represented in the sample to the extent they are present at the site (see TGM Section 5).
Return to the Top of the Page
21.4 ANTICIPATING AND ADDRESSING DATA GAPS
The risk assessor should characterize and address data gaps during the scoping phase of the ERA, as part of the DQO process (see TGM Section 3). A data gap can be generally categorized as resulting from one of two sources: natural variability or incomplete knowledge. A direct evaluation of these types of data gaps can strengthen the DQO process and guide the risk assessor toward a more robust sampling design and a more defensible risk assessment.
The risk assessor should first distinguish between data gaps that result from incomplete knowledge and data gaps that result from inherent variability in the ecosystem. This categorization is based on general knowledge of environmental processes at the site, the CSM, the COPECs, and available data (Table 21-9).
| Table 21-9. Data Gap Analysis |
For data gaps that result from natural variability in the ecosystem, answer the questions below:
|
For data gaps that result from incomplete knowledge about a particular site, chemical, or receptor, answer the questions below:
|
21.5 SUMMARY OF DECISION LOGIC FOR ERAS
The HEER Office applies the decision logic indicated in Tables 21-10 and 21-11, and Figure 21-8 for sediment investigations (see below). It is important to note that the linear flow of the decision tree shown in Figure 21-8 should just be used as a starting point as it is not the only way to approach an ERA. The specific approach should be based on the process outlined in the DQOs and an iterative assessment of meaningful effects, dependent on the particular chemicals and receptors of concern at a site. Many of the items in Table 21-10 and Figure 21-8 are conducted as part of the BERA, such as toxicity testing and tissue sampling. Required, preferred, and optional data for sediment ERAs are summarized in Table 21-11.
| Table 21-10. Questions Guiding Decision Logic for Contaminated Sediment Investigation | ||
| Question | Method | Step |
| Do any chemicals in sediment in the DU exceed HDOH interim No Effect SQGs? | Compare with HEER Interim No Effect SQGs | SLERA Step 2 |
| Could chemicals in prey organisms at the site adversely affect other organisms that consume them? | Evaluate using food chain modeling | SLERA Step 2 |
| Are the chemicals present at concentrations greater than what occur naturally in these sediments or typically in the local environment? | Compare with background/ambient/reference locations | SLERA Step 3A |
| Are the chemicals in a bioavailable form representing exposure to organisms? | Evaluate factors affecting bioavailability | SLERA Step 3A |
| Are organisms at the site directly affected by exposure to chemicals in sediment? | Conduct direct toxicity test or model using representative data | BERA |
| Are organisms at the site bioaccumulating chemicals from the sediment? | Measure field collected organisms or model bioaccumulation using representative data | BERA |
| If yes, could organisms at the site be adversely affected by the chemicals in their tissues? | Evaluate using appropriate tissue effect levels | BERA |
Figure 21-8. Interim Decision Logic for Sediment Investigations in Hawaiʻi
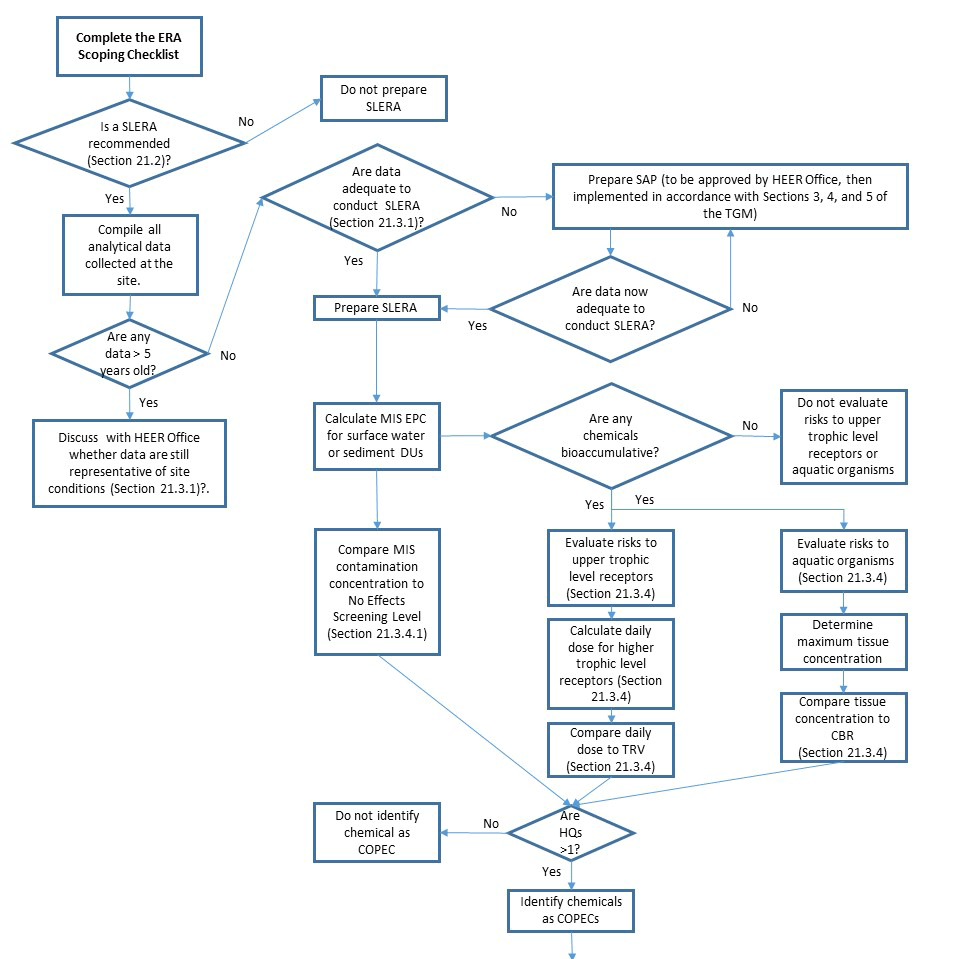
Figure 21-8 (continued). Interim Decision Logic for Sediment Investigations in Hawaiʻi
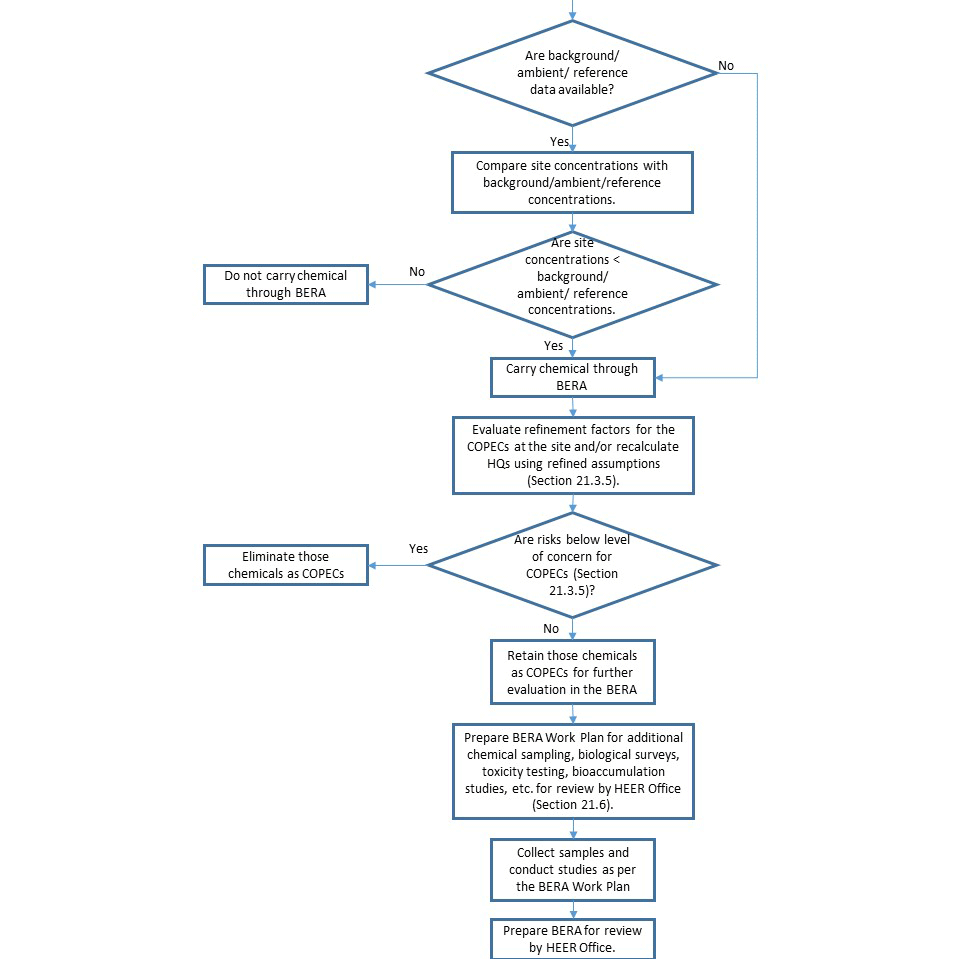
| Table 21-11. Required, Preferred, and Optional Data for Sediment ERAs | |||
| Data Type | Required | Preferred | Optional |
| Sediment (for SLERA or BERA) | |||
| Multi Increment Sediment (MIS) Samples in appropriate decision units (DU) | ● | ||
| Pre-approved Reference Location – all sample types | ● | ||
| Background metals analysis (literature) | ● | ||
| Total 0rganic carbon | ● | ||
| Grain size distribution | ● | ||
| Acid-volatile sulfide and simultaneously extracted metals (AVS/SEM) | ● | ||
| Pore water | ● | ||
| Surface water | ● | ||
| Site-specific tissue (for bioaccumulating chemicals) | ● | ||
| Laboratory Tests (typically just for the BERA) | |||
| Bioaccumulation Test (using native Hawaiian species1) | (if known bioaccumulator is present or suspected) | ||
| Lethal and sublethal toxicity tests using native Hawaiian species | (if one or more chemicals is greater than the Probable Effect SQG) | (if one or more chemicals is between the No Effect SQG and the Probable Effect SQG) | |
| Field-Collected Tissue (typically just for the BERA) | |||
| Field-Collected Tissue (Benthic/epibenthic invertebrate such as crab or octopus; fish species with direct or indirect exposure to sediment) | (if known bioaccumulator is present or suspected) | (in general) | |
| Passive sampling device (for PCBs) | if PCBs exceed No Effect SQG | ||
| 1 If no standard test using a native species is available, provide rationale for a carefully-selected surrogate species | |||
21.6 BASELINE ECOLOGICAL RISK ASSESSMENT
After completing the SLERA, including the Step 3a refinement, the risk assessor is ready to begin Step 4: the BERA. The first task of Step 4 is to prepare a BERA work plan (WP). If additional field data collection is required, the WP may include a field sampling and analysis plan (SAP). Typically, a combined WP/SAP is prepared to streamline the planning and approval process before BERA data collection begins.
The purpose of preparing a BERA WP is two-fold: (1) it compels the risk assessor to thoroughly evaluate existing data, describe site conditions, formulate DQOs, identify data gaps, and anticipate issues that may arise during later risk characterization and data interpretation phases; and (2) it provides a site-specific framework for discussions with the HEER Office during which information can be shared and common goals can be established. This subsection guides the risk assessor through the tasks typically included in the BERA, describes best practices, and reviews technical references to support the process. This subsection assumes that a combined WP/SAP is being prepared. The process of developing the BERA WP/SAP is described below.
- Review the SLERA and ensure that you have access to all available data that contributed to the conclusions of the SLERA.
- Compile any pertinent information collected since the SLERA was prepared. If any new information leads you to question the need for a BERA, present the information and your rationale to the HEER Office for discussion.
- Once you are sure that a BERA is appropriate, prepare a BERA WP/SAP using the outline in Appendix 21-G. The rest of this subsection will provide templates and examples to help you develop the BERA WP.
- Notify the HEER Office that you are preparing a BERA WP/SAP and request additional guidance as needed.
- Submit the draft BERA WP/SAP to the HEER Office well before you expect to begin field work.
As described in previous subsections, the SLERA usually relies on literature-based toxicity and bioaccumulation factors and conservative default assumptions about exposure because site-specific data are not available. The purpose of the BERA is to replace literature or default values with site-specific data so that risk can be more accurately characterized. Site-specific data collection may include toxicity and bioaccumulation tests, collection of organisms, passive sampling of water or sediment, analysis of TOC and grain size, and other types of information. In addition to collection of new data, a more detailed analysis of data available during the SLERA may be warranted.
The components of the BERA mirror those of the SLERA. First, the problem formulation is refined to better describe the environmental setting, ecological receptors, and complete exposure pathways, resulting in a revised CSM (Subsection 2 of the BERA WP/SAP). Then, exposure and effects estimates are updated using site-specific information. The study design for collecting and analyzing new data is in Subsection 3 of the BERA WP/SAP (Study Design and DQOs). Elements of the BERA are presented in Subsections 21.6.1 through 21.6.4 below.
Although each BERA WP/SAP will represent site-specific conditions and address unique considerations, most or all can be prepared using the template in Appendix 21-G. The template provides general direction on which elements should be included in a site-specific BERA WP/SAP and includes useful tips. The HEER Office does not require that the risk assessor follow the template exactly, but it is important that all the necessary components of the BERA be included in the WP/SAP. The full set of topics to be included in the BERA will be determined by the location and geophysical features of the site, the site-specific COPECs, the selected assessment and measurement endpoints, and complete exposure pathways.
21.6.1 BERA REFINED PROBLEM FORMULATION
The problem formulation subsection serves as the “backbone” of the ERA. The SLERA problem formulation (described in Subsection 21.3.3) included a description of the environmental setting, including ecological receptors, potential sources of contamination, and potential exposure pathways, which were used to develop the preliminary CSM. At the start of the BERA, the problem formulation is refined to reflect the conclusions from the SLERA.
The result of Step 3a is a list of COPECs that require further evaluation in the BERA and a list of chemicals eliminated from further evaluation because they were found not likely to cause significant risk. Ideally, the BERA will focus only on chemical-receptor pairs posing potential risk. Careful completion of this step will prevent the risk assessor from wasting time and effort evaluating chemicals in the BERA that should have been screened out during Step 3A.
The refined problem formulation should also identify any data gaps necessary to characterize site-specific risk at the end of the BERA. In some case, information obtained since the SLERA was written may warrant inclusion of chemicals, receptors, or exposure pathways that were not evaluated in the SLERA. For example, the risk assessor may have learned of a historical spill at the site, or a unique habitat with receptors not considered during the SLERA may have been identified. Data gaps identified during review of the SLERA may also require additional lines of investigation. In general, the refined problem formulation should include the environmental setting, COPECs, and assessment and measurement endpoints. Each of these is discussed below.
This subsection of the BERA should describe the environmental setting, COPECs, and sources identified in Step 3a, and ecological receptors. Although much of the site characterization will remain as described in the SLERA, it should be updated with any new information, especially on habitats that will be the focus of the BERA.
<Return to the Top of the Page
21.6.1.1 SEDIMENT DYNAMICS
The SLERA may have relied on assumptions about sediment grain size based on regional geology, as described in the introduction to Section 21. For example, the area may have been described as depositional based on regional data, habitat, or conservative assumptions. For the BERA, it may be necessary to confirm substrate type and grain size at the site to determine whether the area is depositional to better predict chemical behavior and presence of receptors when refining the CSM. Grain size and wave energy must also be considered when selecting an appropriate reference location for the BERA.
Beaches are eroding more than accreting across Hawaiʻi (Fletcher et al. 2012) and coastal erosion is expected to nearly double over the next few decades across the state (except Kailua Beach on Oʻahu). Nevertheless, sediment dynamics are spatially variable, and areas of erosion and accretion may be separated by only a few hundred meters. Each small embayment created by rocky headlands is influenced by local wave energy and terrestrial processes, creating a patchwork of erosion and accretion along the shore. The most recent data on coastal erosion and accretion of shorelines on Kauaʻi, Oʻahu, and Maui are available at (Fletcher et al. 2012). This USGS information should be consulted during the site characterization phase of the BERA.
21.6.1.2 CHEMICALS OF POTENTIAL ECOLOGICAL CONCERN
The list of COPECs developed at the end of Step 3A should include only those chemicals that exceed background or reference concentrations and ecotoxicological effect levels for receptors at the site. If new information suggests the presence of additional chemicals that were not analyzed during the SLERA, then new chemicals should be added to the BERA WP/SAP.
21.6.1.3 ECOLOGICAL RECEPTORS (ASSESSMENT AND MEASUREMENT ENDPOINTS)
Measurement Endpoints)
Based on the results of Step 3a, some receptors considered in the SLERA may be eliminated from further evaluation, and others may be added. The refined problem formulation should include only receptors that will be evaluated in the BERA, based on their known or expected presence at the site or their selection as surrogates for species of interest. The HEER Office has prepared species profiles for selected marine species in Hawaiʻi (Appendix 21-A). The appropriate receptors from this list should be considered for evaluation in the BERA, noting that additional exposure information may be needed to quantify risks to some receptors. Note that the list of species in Appendix 21-A is not comprehensive; other species may be evaluated in the BERA if approved in advance by the HEER Office. In the BERA WP/SAP, explain any changes to the list of receptors in the SLERA.
Assessment and measurement endpoints that are commonly evaluated in marine sediment ERAs are summarized in Table 21-5 (see Subsection 21.3.3). This subsection of the BERA should provide rationale for the selected assessment endpoints and describe how each assessment endpoint will be evaluated using the selected measurement endpoints. A table similar to Table 21-5, including the following elements, should be developed for the BERA:
- Ecological Guild: The functional niche of the receptor (such as benthic invertebrate)
- Assessment Endpoint: The specific attributes of value for the ecological guild at the organism or population level.
- Species Evaluated: Table 21-8 lists typical species included in each ecological guild. In the BERA, identify the species that were used to represent the ecological guild, along with the rationale for selecting the species. In some cases, species other than those listed in Table 21-8 may be used based on available data. Use of other species should be presented in the BERA WP/SAP and approved in advance by the HEER Office.
- Measurement Endpoint: Table 21-5 lists common measurement endpoints for each of the assessment endpoints. In the BERA, present the specific measurement endpoints that were used to evaluate the assessment endpoints, along with the rationale for selecting those endpoints. The measurement endpoints may include some or all the endpoints listed in Table 21-5, and endpoints not listed in the table that are deemed appropriate for the site.
21.6.1.4 REFINED CONCEPTUAL SITE MODEL
The screening level CSM was developed as part of the SLERA based on what was known about the site at that time, without regard to potential ecological risks. As described in Step 1b, Task 5 (Subsection 21.3.3), the elements of the CSM include (1) ecological receptors present at the site; (2) sources of chemicals in the environment; (3) contaminant transport pathways; and, (4) exposure pathways to the ecological receptors. The same elements are included in the refined CSM, which represent the chemicals, receptors, and exposure pathways evaluated in the BERA.
Appendix 21-C describes the approach for defining the ecological DU. DUs set the boundaries for where the BERA investigations will be conducted. The refined conceptual site model should describe the DUs that were selected for each assessment endpoint evaluated in the BERA and the rationale for selecting them. Refer to the discussion of sediment types at the beginning of Section 21 before identifying DUs. Note also that the size of the DUs is determined in part by the receptors, as home range is an important variable in the evaluation of exposure and effects. The site may contain several DUs designated by sediment type, wave energy, preliminary contaminant concentrations, receptor distribution, and other factors.
21.6.2 BERA STUDY DESIGN AND DATA QUALITY OBJECTIVES
The BERA should describe the investigations conducted to evaluate each assessment endpoint, such as chemical analysis, toxicity testing, bioaccumulation studies, biological surveys, and tissue analyses. The DQO process that was followed during the SLERA (see TGM Sections 3, 4, and 5) should be revisited when preparing the BERA WP/SAP. The study design and DQOs should be presented in the BERA WP/SAP and cited in the BERA. Because the BERA WP/SAP will be included as an appendix to the BERA, it is not necessary to repeat the DQO subsection. A Quality Assurance Project Plan (QAPP) should also be prepared as part of the BERA planning effort (see TGM Section 10).
21.6.2.1 LABORATORY ANALYSES
Additional data collected for the BERA are likely to include field samples of sediment, sediment pore water, surface water, groundwater, or even soil (in case where terrestrial erosion is suspected as a transport pathway to the marine site). The BERA WP/SAP should identify analytical methods and detection limits to ensure that detection limits lower than selected screening levels can be achieved.
The HEER Office recommends evaluating chemicals with similar modes of toxicity as “total” concentrations, but analysis of individual constituents may also be necessary. Total concentrations are commonly calculated for HMW PAHs, LMW PAHs, total PAHs, total PCBs, DDT and its breakdown products (total DDTx), and dioxin toxic equivalency quotients (TEQs). Methods for calculating total PCBs and dioxin TEQs are discussed later in this subsection, but the risk assessor is encouraged to review the current literature and determine the most appropriate method for the site. No specific list of constituents or summation method is prescribed because methods are rapidly changing as new technical literature is published, methods are vetted, and best practices are disseminated within the risk assessment community. The BERA WP/SAP should describe the proposed methods of summing constituents and clearly identify the individual constituents to be included in the sum. Relevant literature should be cited to support the proposed methods.
In general, HDOH requires the following when calculating total values:
- Non-detected values should be assigned a value of zero provided the detection limits were acceptable, as described above.
- The mean of replicate samples (i.e. triplicates or duplicates) should be used for the calculation.
- The list of individual constituents included in the total calculations must be given (e.g. see notes at the bottom of Table 21-7 for a list of HMW and LWM PAH totals).
Risks from dioxins/furans should be evaluated by using Toxicity Equivalence Factors (TEFs) to calculate toxicity equivalence concentration (TEQ) as described in the Framework for Application of the Toxicity Equivalence Methodology for Polychlorinated Dioxins, Furans, and Biphenyls in Ecological Risk Assessment (USEPA 2008g). The detected concentration of each dioxin (or furan) in a sample is multiplied by its TEF. The resulting values for each sample are summed to calculate the TEQ Dioxins/Furans for each sample. TEQs should be calculated for birds, mammals, and fish using chemical-specific TEFs for each group; no dioxin TEFs are available for plants and invertebrates.
PCB results historically have been reported as Aroclors (i.e., Aroclor-1254, Aroclor-1260) in BERAs because the early ecotoxicological studies were based on total PCBs expressed as the sum of Aroclors. Although some current studies continue to report effects of total PCBs, newer literature is increasingly focused on one or a small set of the 209 PCB congeners. Each Aroclor originally contained a specific combination of PCB congeners and could be identified by its distinctive chromatographic pattern when is analyzed by gas chromatography. However, as Aroclors age and weather, the chromatographic patterns may change and not be recognizable as standard patterns. Such degradation of Aroclors may cause the laboratory to underestimate the concentration of total PCBs in a sample. (See USEPA 2013c) for more detail on this issue.
Analysis of PCB congeners is considerably more expensive than Aroclors, so the decision of analytical method must be made with care. The HEER Office recommends that PCBs be analyzed as Aroclors during the SLERA. However, if total PCBs are detected at concentrations exceeding the screening level in the SLERA samples, a subset of samples (no less than 10 percent) should be analyzed for all 209 congeners. Note that twelve of the PCB congeners have been designated by the World Health Organization (WHO) as having “dioxin-like” toxicity (Van den Berg et al. 1998). The same process described above to calculate the TEQs for dioxins (USEPA 2008g) can be used to sum the dioxin-like PCBs when site conditions warrant. The BERA WP/SAP should describe the rationale for the selected analytical methods for PCBs (Aroclors, congeners, or a combination of the two) and discuss how the dioxin-like PCBs will be summed if samples are analyzed for PCB congeners.
21.6.2.2 SEDIMENT SAMPLING
The objectives of the study and availability of existing data play an important role in dictating the sampling design, methods, and equipment. For example, MI sampling should be conducted to determine representative average contaminant concentrations in sediment across a designated DU (see Sections 3, 4, and 5. Subsection 5.7 of the TGM (Sediment Sampling) discusses issues affecting sediment sampling in more detail.
A wide variety of sampling equipment is available for collecting sediment, but not all equipment is suitable for all sites. For example, grab samplers such as a ponar dredge or Van Veen grabs are capable of sampling only the top several inches of sediment, while sediment corers and vibracores can be used to collect deeper samples if historical chemical concentrations are needed. Other considerations include whether the sediment sample must be undisturbed (as it should be for analyzing volatile organic compounds). Water depth, currents, sediment volume, bottom firmness, and other parameters also influence the likelihood of success of each collection method. When acid volatile sulfides [AVS] are to be analyzed, exposure of the sample to oxygen must be limited. A thorough discussion of the various sediment sampling devices, including advantages and disadvantages of each and the best samplers to use for different types of sediment is presented in Chapter 3 in Methods for Collection, Storage and Manipulation of Sediments for Chemical and Toxicological Analyses: Technical Manual (USEPA 2001i). The BERA WP/SAP should include a complete description of equipment, techniques, and standard operating procedures (SOPs) for all sediment collection methods; references should be cited as needed to support the proposed methods.
The BERA WP/SAP should describe the procedures for any representative sub-sampling of sediment samples in the field. This is a critical component of sample processing and should be based on the objective of the investigation, the COPECs, and the sediment matrix. Typically, processing and representative sub-sampling of MI samples are conducted in the laboratory following an established SOP (see TGM Section 4).
Sediment samples must be collected from the appropriate depth to address the goals of the BERA (as identified in the DQO analysis). General guidance on selecting the appropriate depth for collecting sediment samples in the biologically active zone is in Determination of the Biologically Relevant Sampling Depth for Terrestrial and Aquatic Ecological Risk Assessments (USEPA 2015e). Table 21-12 summarizes the depths of the biotic zone associated with different sediment substrates and lists habitats in Hawaiʻi that may contain that substrate.
| Table 21-12. Typical Depths of Biotic Zone | |||
| Depth | Sediment Substrate | Example Habitat Type | |
| 5 cm | oligohaline/polyhaline mud | Mudflats | |
| 5 cm | oligohaline sand and marine coastal sand | Sandy Beach | |
| 10 cm | marine coastal mixed and marine offshore sand | Seagrass beds | |
| 10 to 15 cm | estuarine and tidal freshwater environments | Stream-fed Estuarine Wetlands | |
The HEER Office recommends taking the above-referenced guidance into consideration when determining appropriate sampling depths to capture the biotic zone. However, depending on the objective of the investigation, deeper samples (below the biotic zone) may also be needed to characterize vertical extent of contamination.
Special sediment sampling consideration may be warranted for target receptors that ingest sediment directly, as sediment effect levels may not account for the ingestion pathway. Ingestion is the basis for the food chain modeling used to evaluate risk to birds and mammals, but many benthic invertebrates and fish also consume sediment as part of a typical diet. Tissue concentrations of benthic invertebrates may reflect chemicals adsorbed to ingested sediment particles as well as chemicals absorbed directly from sediment and water (Lee et al. 2006; Belzunce-Segarra et al. 2015). To evaluate the sediment ingestion pathway, sample collection methods must ensure that the top layer of fine particles is retained for analysis.
When developing the BERA WP/SAP, sediment sample collection log sheets from the SLERA should be reviewed to determine whether they contain useful information to guide the BERA. For example, if sulfide odors were detected during sediment sampling, then AVS may be present in the sediment. Methods for evaluating bioavailability of metal mixtures in sediment containing AVS are discussed in Procedures for the Derivation of Equilibrium Partitioning Sediment Benchmarks (ESBs) for the Protection of Benthic Organisms: Metal Mixtures (Cadmium, Copper, Lead, Nickel, Silver, and Zinc) (USEPA 2005e).
At some sites, it may be appropriate to use a Dynamic Sampling Approach, in which field analytical methods such as x-ray fluorescence (XRF), immunoassays, or other mobile screening approaches help make quick decisions regarding the need to collect samples in a location. This approach is discussed briefly in Subsections 3.10 and 5.5.8 of the TGM. Basically, this approach allows samples to be collected and sites to be characterized more efficiently and quickly than traditional sampling. The costs and benefits of a dynamic sampling approach should be discussed in the BERA WP/SAP. See A Guideline for Dynamic Workplans and Field Analytics: The Keys to Cost-Effective Site Characterization and Cleanup (Robbatt 1997 and USEPA 1997i) for more information on field assessment techniques.
21.6.2.3 PORE WATER SAMPLING
In sediments where sediment pore water is relatively static, contaminants in the pore water are expected to be at thermodynamic equilibrium with the sediment (solid phase), making pore water useful for assessing contaminant levels and associated toxicity (USEPA 2001i). The utility of collecting sediment pore water at a site is influenced by a variety of factors, including the solubility of the chemicals, ongoing sources of chemicals in groundwater, grain size and organic content of sediment, and other factors. Sites where pore water analysis may be appropriate include fine-grained sediments in low energy depositional areas (such as bays and harbors) and nearshore sites where contaminated groundwater is known or suspected to discharge to sediment. The suitability of sediment pore water as an exposure pathway to ecological receptors should be evaluated as part of the DQO process and documented in the BERA WP/SAP.
Pore water collection methods should be tailored to the site and the contaminants of concern. No single method is clearly superior in all cases. For example, peepers are suitable for collecting small volumes of pore water for one or two analyses but are not practical for collecting large volumes required to analyze for numerous chemicals. Fine-grained sediments may be collected in buckets and taken to the laboratory for extraction of pore water by centrifugation. However, centrifugation may overestimate concentrations of freely dissolved contaminants (Cfree) in sediment porewater. Depending on the target receptors, the freely dissolved concentration may be a more appropriate exposure point concentration than the total concentration in pore water. Pore water samples also can be collected directly from the sediment using drive points and pushpoint samplers (Henry samplers). In coarser-grained sediments, especially where contaminants are being continuously discharged, in situ measures may be more practical because coarse grained sediment does not retain much water when collected. Traditional collection of sediment followed by centrifugation would require substantial effort because of the large volume of sediment needed to yield an adequate volume of pore water.
Passive in situ sampling methods may be suitable in cases where collecting large volumes of sediment for centrifugation is impractical. or when other limitations of centrifugation are of concern. For example, when chemicals of concern are volatile or unstable, concentrations in pore water may change as the sediment is transported to the lab and centrifuged.
Pore water in situ sampling methods for coarse-grained sediments are under development. The Laboratory, Field, and Analytical Procedures for Using Passive Sampling in the Evaluation of Contaminated Sediments: User’s Manual (USEPA/SERDP/ESTCP. 2017) provides the most comprehensive review of methods for passive sampling of contaminated sediments. The manual provides guidance on selecting and implementing passive sampling technology to evaluate PCBs, PAHs, and selected metals (cadmium, copper, nickel, lead and zinc) in sediment. Earlier technical reviews of passive sampling are provided in (Ghosh et al. 2014), (Greenberg et al. 2014), (Lydy et al. 2014), (Mayer et al. 2014), and (Peijnenburg et al. 2014).
Passive sampling consists of inserting various materials such as polydimethylsiloxane (PDMS), low-density polyethylene (LDPE), or other similar materials into the sediment for a period of time (usually several weeks or months). The materials are typically mounted on frames and may be enclosed by screens or tubes for protection. The samplers are cleaned with an organic solvent to remove oligomers, plasticizers, and contaminating organic chemicals prior to deployment in the field. In some cases, performance reference compounds (PRCs) are added to the sampler as a quality control for estimating the extent of equilibrium of the target contaminant. After the samplers are retrieved from the sediment, the sampling material is cleaned, the contaminants are extracted, the extract is analyzed for contaminants, and concentrations of cfree are calculated. The sampler can be sectioned prior to extraction, if desired, to investigate vertical concentration gradients.
The BERA WP/SAP should specify the methods of collecting and analyzing sediment pore water will be used and provide rationale for selecting the methods. It is essential that the same collection procedures be used and the pore water be collected at the same depth across the site so that appropriate comparisons can be made (USEPA 2001i). Likewise, the same methods must be used at the reference location. If the pore water concentrations will be compared with water quality criteria, the WP must specify how the cfree concentrations will be interpreted with respect to the dissolved criteria for protection of aquatic life. In some cases, side-by-side analysis of standard dissolved concentrations may be required to establish that the passive sampling methods are representative. Additional methods are discussed in several comprehensive technical references:
- USEPA 2001i: Methods for Collection, Storage and Manipulation of Sediments for Chemical and Toxicological Analyses: Technical Manual (Chapter 6).
- Carr et al. 2001: SETAC Technical Workshop on Porewater Toxicity Testing: Biological, Chemical, and Ecological Considerations with a Review of Methods and Applications, and Recommendations for Future Areas of Research
- Various authors 2014: “Passive Sampling Methods for Contaminated Sediments,” in the SETAC Technical Workshop “Guidance on Passive Sampling Methods to Improve Management of Contaminated Sediments” in Integrated Environmental Assessment and Management (Volume 10) reviews the use of passive samplers to quantify concentrations of chemicals in sediment pore water. (Ghosh et al. 2014, Greenberg et al. 2014, Lydy et al. 2014, Mayer et al. 2014, Parkerton et al. 2014, and Peijnenburg et al. 2014).
21.6.2.4 SURFACE WATER SAMPLING
The surface water pathway is evaluated by comparing chemical concentrations in surface water with water quality standards based on ecotoxicity. However, surface water should be evaluated only in places where the water has a relatively long residence time so that the exposure duration is meaningful. For example, surface water is not considered a measurable pathway at sites where high energy wave action mixes the water constantly. The HEER Office generally does not recommend collecting surface water samples from high energy environments or areas where considerable flushing occurs. In contrast, surface water could be an important pathway in a protected bay contaminated by a surface release, stream input, or groundwater flow. Surface water samples should be collected if chemicals in groundwater are known or suspected to discharge through sediment into protected surface water areas.
Surface water samples may be analyzed for total or dissolved chemicals, depending on the proposed use of the results. Samples that will be compared with the HEER Office EALs for aquatic life for metals should be analyzed for dissolved fractions, represented by samples passed through a 0.45 micrometer (μm) filter. The filtering step typically takes place in the lab, although field-filtering is an option under special circumstances. The USGS provides comprehensive guidance on proper methods for collecting water samples in the National Field Manual for the Collection of Water-Quality Data: U.S. Geological Survey Techniques of Water-Resources Investigations, Book 9, Chapters A1-A10 (USGS 2018).
Both freshwater and saltwater (marine) standards are available. Freshwater and saltwater standards apply to waters with a dissolved inorganic ion concentration less than and greater than 0.5 parts per thousand (ppt), respectively. Saltwater samples can be analyzed for dissolved constituents only. In freshwater habitats, however, total concentrations from unfiltered samples are better indicators of the concentrations ingested by animals as drinking water and are preferred as inputs to the food chain model (see Step 2, Task 3). Freshwater samples may be split and analyzed as both total and dissolved concentrations. The BERA WP/SAP should clearly indicate and provide rationale for which water quality standards will be applied and which water samples will be filtered.
Sample numbers and locations, sampling equipment, and proposed analyses should be presented in the BERA WP/SAP. Equipment should be selected based on the depth of water to be sampled, volume of water needed, strength of currents, and other logistical factors. For example, if the objective is to collect surface water samples at the surface water-sediment interface to determine whether groundwater discharge is transporting chemicals to surface water, a horizontal water bottle sampler may be appropriate. Alternatively, passive sampling devices can be deployed at the sediment-water interface to measure concentrations over time in a specific area. Passive sampling devices are newer and less standardized but may be acceptable for use at some sites. Regardless of the methods and equipment selected, it is important that site samples and reference area samples be collected in the same way.
The BERA WP/SAP should present a rationale for the selection of devices, equipment, and methods. The procedure should be designed so as to minimize incidental collection of suspended solids with the water sample, as solids can artificially inflate measured chemical concentrations. Such interference can be especially important when relatively hydrophobic chemicals such as PCBs and pesticides are being analyzed. In such cases, side-by-side analyses of filtered and unfiltered samples may be warranted.
21.6.2.5 BIOLOGICAL SURVEYS
Biological surveys may be conducted as part of a BERA for many reasons:
- To document the presence and abundance of ecological receptors at the site, including protected or rare species;
- To compare the distribution or abundance of species with reference areas or historical records;
- To evaluate the health or integrity of the ecological community; and
- To collect tissue samples for chemical analysis (described below) for use in food chain models or critical body residue analyses.
Field surveys may be designed for the reasons listed above, as well as simply to ground-truth the CSM. Unlike sediment and water sampling, which may be conducted by general field teams, biological surveys should be conducted by experienced biologists or ecologists who are prepared to document and interpret what they see in the field. Although a single species or type of organism may be targeted for collection, the presence and condition of other species may inform the BERA. Well-designed biological surveys focus on structured data collection, but a competent field biologist will also make opportunistic findings, such as the presence of unanticipated species; the relative scarcity of individuals where abundance was expected; evidence of degraded habitat such as algal overgrowth, stressed vegetation, or chemical sheens and odors; and other features that are not the direct target of the survey.
The BERA WP/SAP should describe the proposed survey as thoroughly as possible, including but not limited to the elements below:
- Objectives of the survey
- Qualifications of the field team
- Locations to be surveyed (with rationale), and process for adjusting the location when field conditions warrant
- Relation of survey locations to established DUs
- Intended dimensions of each survey location (length and width)
- Survey methods (areal grid, transect, etc.)
- Sample field forms
- Protocol for avoiding habitat degradation during survey
- Protocol for unintended encounters with protected species
- Temporal requirements of the survey: time of day, season, restrictions based on weather
- Health and safety issues (to be documented in a separate health and safety plan)
- Use of survey data (species richness, taxonomic diversity, percent dominant taxa, frequency and dominance of stressor tolerant taxa, etc.)
Surveys at the site should be repeated to the extent feasible at reference locations. The reference locations should be similar in size, substrate (grain size distribution), wave energy, surrounding habitat/land use (i.e. urban, rural, forested, etc.).
21.6.2.6 FIELD-COLLECTED TISSUE SAMPLING
Organisms may be collected from the site (and reference location) during a biological survey or as a separate activity. Field-collected organisms may contribute in several ways to the BERA:
- Whole organisms or body parts may be analyzed for selected chemicals. When appropriate, chemical concentrations in the organisms can be compared with concentrations in sediment to evaluate bioavailability and uptake by the organism. Note that this approach requires that both the organisms and the sediment be relatively immobile.
- Organisms may be collected as part of a biological inventory focused on characterizing the health of the community in a given area. Species distribution and abundance, species diversity, age or size class distribution, reproductive condition, and other parameters may be measured.
- Organisms may be collected for evidence of disease, which may then be linked to chemical contaminants in the sediment or water. External tumors or lesions may indicate exposure to PAHs, for example. Internal examination may reveal parasites, liver damage, or other evidence of degraded health.
Note that a Special Activity Permit may be required for collecting marine organisms for the BERA, even if the organisms are returned to the water unharmed. The Hawaiʻi Division of Aquatic Resources should be contacted during the BERA planning stages to identify necessary permits (DLNR DAR 2018). Other permits may be required for collecting protected species or certain native species, or for collecting in parks or other specified areas. The risk assessor should coordinate with the Hawaiʻi Division of Forestry and Wildlife (DLNR DFW 2018) to obtain the required permits.
In addition to the elements listed above for biological surveys of any kind, the BERA WP/SAP should fully describe the proposed rationale and methods for collecting and analyzing organisms, including at a minimum the following details:
- Objective of the collection effort
- Target species to be collected and alternate species in the event that the target species cannot be collected
- Locations, relative to DU, and protocol for field adjustment of locations
- Number of individuals of each species to be collected (by sex and size, if relevant), per location, including reference location
- Number of organisms to be composited in each sample (single species only)
- Body part(s) to be tested (whole body, liver, eggs, blood, etc.)
- Other parameters to be measured (lesions, parasites, fin rot, etc.)
Selection of Appropriate Species for Field Collection
Selecting the appropriate species for field collection is critical to the defensibility of the BERA. Not all species are suitable for answering all questions. The three principal reasons for collecting organisms from the site are (1) chemical analysis; (2) community metrics; and (3) evidence of disease.
All three of these lines of evidence require species with the following characteristics:
- Exposure: The species is exposed to the site (and the reference area) for a substantial period of time relative to its lifespan, so that observed effects can be linked to the site. Year-round residency is desired but not required.
- Ecological Relevance: Organisms should be ecologically relevant to the evaluation. For example, if risk to the wedge-tailed shearwater from fish consumption is being evaluated, individual fish of the appropriate species and size should be collected. Seasonality should also be considered (see below).
- Abundance: Field-collected species should be abundant enough at the site and reference area to support collection of specimens for the intended use. See Table 21-13 for tissue volumes generally required for chemical analyses.
| Table 21-13. Typical Tissue Volumes Required for Selected Chemical Analysis | |||
| Chemical Group | Tissue Volume Required (grams wet weight) | ||
| Low Level Detection | Standard Level Detection | ||
| Metals | 2 | 2 | |
| Pesticides | 15 | 1.5 | |
| PCBs | 15 | 1.5 | |
| Dioxins/Furans | 10 | 10 | |
| SVOCs | 30 | 2 | |
| Percent Lipids and Moisture | 10 | 3 | |
Species collected specifically for chemical analysis must meet the following additional criteria:
- Ability to accumulate the chemical: Many metals are accumulated by both plants and animals, but most organic chemicals are not likely to be accumulated in plants. Metals that are essential nutrients may be actively regulated by the organism and thus not suitable for use as indicators of bioavailability. Verify that the COPECs being evaluated are known or expected to accumulate in the organism targeted for collection.
- Limited ability to metabolize the chemical: Some organisms metabolize certain organic chemicals, which makes the compounds less likely to accumulate in tissues. For example, PAHs induce mixed function oxidase enzymes (and thus their own biotransformation) in fish and other vertebrates, but not in mollusks or crustaceans (USEPA 2000i). Although fish may show signs of PAH exposure, such as lesions or tumors, tissues may not contain elevated concentrations of PAHs relative to sediments.
- Sex and Seasonal Variability: Chemical concentrations in a species may vary by sex, often influenced by reproductive processes. For example, a female fish or invertebrate may transfer some organic chemicals to her eggs, thus reducing her body burden. Chemical analysis of composite samples made up of several individuals may vary from one another simply because the sex ratios in the samples differed. This situation would confound the analysis of site-related bioavailability and compromise the findings of the BERA. Whenever possible, the sex and reproductive condition (pre- or post-spawning) of individuals in a composite (and across the site and reference area) should be matched. Likewise, chemical concentrations in organisms may vary by season. A study of tissue concentrations at Ordnance Reef reported that metals were higher in goatfish samples in the fall, but higher in octopus samples in the spring. The BERA WP/SAP should include a review of published findings on factors affecting seasonal variability to support the proposed sampling approach.
Collection and analysis of organisms can be time consuming and costly, as well as potentially affecting the habitats and communities at the site and reference area. The rationale for tissue collection should be clearly explained in the BERA WP/SAP so that the most appropriate organisms are collected to address the study objectives. Appendix 21-A presents profiles of 22 common Hawaiian species, including information on previous tissue analysis. The HEER Office recommends that these 22 species be used whenever possible so that a more robust statewide dataset can be developed.
Tissue Sample Handling and Processing
The BERA WP/SAP should describe methods for handling and processing field-collected organisms, including preservation (freezing or refrigerating), dissection (body parts to be analyzed), homogenization techniques, and other procedures. No single approach is appropriate for all tissue samples. If the tissue concentrations will be used in a food chain model, then the whole body should generally be analyzed. If a COPEC is known to differentially accumulate in a single organ, such as the liver, then an organ-specific analysis may be more appropriate. In some cases, only a part of an organism (blood, eggs, feathers) is collected.
The approach to preparing laboratory analysis replicates of tissue samples should be described in the QAPP. In most cases, replicate samples for tissue samples (i.e. triplicates or duplicates) will be prepared by the laboratory after the sample is homogenized and replicates need to be collected in separate random locations across the homogenized tissue, not co-located. Separate samples collected in the field, even from a single location, are considered different samples for analysis, not replicates.
In most cases, the HEER Office recommends that the laboratory report the results in dry weight and also measure and report percent moisture. Various uses of the results may require wet weight or dry weight concentrations. For example, if the results will be used as inputs into a food chain model, and the predator’s ingestion rate is on a dry-weight basis, then the results should be in dry-weight. However, if the tissue results will be compared to critical body residues that are presented in wet-weight, then the results should be presented in wet-weight. In either case, if percent moisture in the tissue samples is reported, concentrations can be converted between wet-weight and dry-weight by the risk assessor as needed. Percent lipids should also be measured in any tissue analyzed for organic chemicals.
Spatial Correlation with Sediment Samples
As mentioned above, tissue concentrations can provide a strong line of evidence for bioavailability and potential toxicity of chemicals in sediment. However, the strength of this line of evidence is dependent on the degree to which the organisms are linked to the area of known sediment concentrations. It is essential that tissue samples be co-located in space and time with sediment samples, and that both are relevant to the DUs previously established.
Collection of Reference Samples
Because most sites are affected by general human activity apart from any site-related chemical release, the use of reference locations is essential to a strong ERA. Reference samples are used as a basis of comparison so that site-related chemical concentrations can be interpreted in the context of ambient or background chemical concentrations. The designation of reference areas was discussed previously (Step 3A, Task 1 in Subsection 21.3.4 and Appendix 21-E, Step 3). The HEER Office is compiling a database of tissue concentrations collected across the state for numerous purposes. During the BERA WP/SAP review process, the HEER Office may make these data available to the risk assessor to support a more robust analysis of ambient tissue concentrations.
The HEER Office must approve the reference area prior to sample collection. A minimum of three tissue samples should be collected at the reference site, using the following guidelines:
- Reference samples should be of the same species, size (+20%), and sex as the site samples.
- Site samples should be collected first, followed immediately by reference samples. This ensures that the reference species can be matched to the site samples.
- Reference areas should reflect general regional conditions (air deposition, general land use) but not be affected by site contaminants or other known sources of contamination. Physical habitat must be comparable to the site (wave energy, grain size, salinity, etc.)
21.6.2.7 TOXICITY TESTING
Chemicals detected in sediment, surface water, or pore water are not necessarily in a form that can cause adverse effects on receptors. To directly measure the bioavailability and potential toxicity of a sample, a test organism is exposed to the sample under controlled conditions. Laboratory bioassays are used to test the reactions of living organisms to water or sediment collected from a potentially contaminated site. Because interpretation of in situ field bioassays using native organisms can be confounded by multiple factors, standardized laboratory bioassay tests with a small number of well-studied species are typically used instead. Whether the suite of bioassay organisms and the particular test protocols that have become the norm in the mainland U.S. are reasonable tools for tropical marine assessments has been the topic of discussion during the past 20 years (Peters et al. 1997; Batley and Simpson 2008; Simpson et al. 2007).
Need for specific protocols to address ecological risk in tropical marine ecosystems was identified during the early stages of the USEPA ERA framework process because differences in the geochemistry and physical nature of sediment, climatic conditions, and other features of tropical ecosystems suggest that the exposure pathways may not be adequately represented by protocols developed for temperate ecosystems. Tropical marine ecosystems are not well represented by standard USEPA bioassays or exposure models.
Since that initial review paper (Peters et al. 1997), which thoroughly described the steps necessary to develop a tropical marine program, progress has been slow. Despite substantial advances in assessing ecological risk in general, the focus is still on temperate ecosystems (Batley and Simpson 2008).
Tropical marine species can be substituted for temperate species in some cases. Examples of new bioassay protocols developed to address tropical regions of Australia include the following (based on Adams and Stauber 2008):
- Tests using native benthic unicellular microalgae measure enzyme activity rather than growth; the test can be used in a wide range of grain sizes.
- A native polychaete (Scoloplos sp.) was substituted in an ASTM method; the native polychaete is an infaunal tunneler that lives in sediment of a wide range of grain sizes.
- No tropical amphipod test has been developed, but these authors suggest that amphipods exposed to typical coarse-grained sediments of coralline habitats may have to be fed during the test. (Tests with the freshwater Australian amphipod Melita plumulosa were not compromised by feeding.)
- The tropical hermit crab (Diogenes sp.) can be used for whole sediment bioassays. (Although this genus may not occur in Hawaiʻi, other members of the Family Diogenidae may be equally useful as test organisms.)
- A standard bivalve bioassay can be modified to use the widespread tropical Donax cuneata.
The Sediment Evaluation Framework for the Pacific Northwest (Northwest Regional Sediment Evaluation Team (RSET 2016) provides a useful overview of toxicity testing and approaches for evaluating the results. The HEER Office continues to work with researchers to identify appropriate test organisms for contaminated marine sediment sites in Hawaiʻi. The BERA WP/SAP should provide rationale for the proposed toxicity tests, including and modifications to standard protocols that would make the tests more representative of site conditions (water temperature, day length, etc.). The HEER Office will discuss other options with the risk assessor as needed.
In addition to the test species, the BERA WP/SAP Work Plan should describe the overall approach to the toxicity tests, including the duration of exposure; feeding regime; endpoints evaluated (growth, survival, reproduction, other); number of replicates; parameters measured during the test and frequency of measurements (pH, ammonia, other); and other specific test procedures. The criteria for sample selection should also be described. Issues that must be considered in the design of toxicity test samples include, but are not limited to, those below:
- What is the purpose of the toxicity test? What is the null hypothesis?
- Will toxicity testing run concurrent with or after chemical analysis?
- If chemical results are known, will samples for toxicity tests be selected randomly or purposefully to represent a range of concentrations?
- If purposefully selected, how will concentration bins be determined? What if more than one chemical is detected at the site (the most typical situation)?
- What types of correlation or regression analyses are planned? How many samples are required for robust analysis?
- How will variability among endpoints be interpreted? (For example, the test may show no effect on growth but a significant decrease in reproductive output, or vice versa.)
- How will samples form the reference area be selected?
- How will toxicity in site samples be evaluated with respect to the reference area?
The questions above, and any other relevant issues, should be thoroughly discussed in the BERA WP/SAP. Well-designed toxicity tests can provide a strong line of evidence to the BERA, but poorly designed tests waste time and money while only adding to the uncertainty in the BERA.
21.6.2.8 LABORATORY BIOACCUMULATION TESTING
Several limitations with field collected organisms can be addressed by conducting laboratory bioaccumulation tests. For example, while field collected organisms can answer questions about exposure to chemicals in the wild, it is never possible to identify with certainty when or how the chemicals were taken into the organism’s tissues. In other cases, organisms may be too scarce or difficult to collect from the site. Laboratory bioaccumulation tests also have disadvantages, such as using test organisms that are not native to the site, misrepresenting conditions in overlying water at the site, and interrupting normal feeding habits of the test organisms. Even when the same species is tested in the field and in the laboratory, results may vary. For example, tests comparing bioaccumulation in an estuarine bivalve (Tellina deltoidalis) under lab and field conditions reported that important parameters differed between lab and field over the 31-day exposure period. Percent fines at the surface of the test sediment increased in the field but not in the lab. AVS increased in lab but not in the field (Belzunce-Segarra et al. 2015). This and other studies serve as a caution against extrapolating or over-interpreting both lab and field results. Despite these caveats, laboratory bioaccumulation tests can provide an independent line of evidence to the ERA. The Sediment Evaluation Framework for the Pacific Northwest (Northwest Regional Sediment Evaluation Team (RSET 2016) provides a useful overview of bioaccumulation testing.
The proposed laboratory bioaccumulation test should be thoroughly described in the BERA WP/SAP, referencing protocols when available. Include information on the exposure duration, test organisms, depuration, parameters measured during the test, frequency of measurements, endpoints, replacement of overlying water, feeding, and any other variable that could affect the usefulness of the test. The BERA WP/SAP should describe how samples will be selected for bioaccumulation testing, in keeping with the discussion above for toxicity tests.
Prior to initiating the test, at least one representative tissue sample of test organisms must be collected and either immediately analyzed or frozen for analysis with the test samples after the test is completed. This sample will serve as the baseline concentration for comparison of test samples.
Depending on the study objective, organisms may or may not be depurated to eliminate sediment from the gut prior to chemical analysis. When the goal of the test is to derive a BSAF, or to compare bioaccumulation among several samples, then the organisms are typically depurated. If the goal of the bioaccumulation test is to provide concentrations in prey organisms for use in the food chain model, then the test organisms should not be depurated. The rationale for depurating (or not) should be given in the BERA WP/SAP.
After the exposure period, test organisms are processed and analyzed for chemical constituents. The BERA WP/SAP should provide details on which samples (if not all) will be analyzed, how they will be homogenized, whether they will be frozen or otherwise preserved, and which analyses will be performed.
As mentioned above, tissue analytical results should be reported as dry weights. Percent moisture and percent lipids should be measured whenever organic compounds are analyzed. The BERA WP/SAP should specify how tissue results will be interpreted with respect to laboratory controls and reference area samples. For example, what does it mean when tissue concentrations at the site are 10 times concentrations at the reference area?
21.6.3 DATA ANALYSIS AND INTERPRETATION
The results of the chemical sampling, biological surveys, toxicity testing, bioaccumulation studies, and any other data collected are evaluated in the data analysis subsection of the BERA. The HEER Office expects that the risk assessor will follow current practice and adhere to professional standards in analyzing and interpreting the data. If the risk assessor is not familiar with the general process of preparing an ERA or would like a review, numerous publications available to the public offer guidance and assistance on specific topics. Current USEPA ERA guidance can be accessed online (USEPA 2018b). Older ERA guidance documents have been made easily accessible by Oak Ridge National Laboratory (ORNL 2018).
In general, all field-generated data and records (such as the field data sheets) should be reviewed for completeness and accuracy by the risk assessment technical lead. All field-generated data, including photographs and videos, should be maintained in the project file and included (as appropriate) in the final BERA. Notes on selected topics important to the HEER Office are presented below. The risk assessor should contact the HEER Office to request additional support if needed.
21.6.3.1 FIELD NOTES
Descriptions of the sediment, surface water, and habitat such as odors, colors, sheens, debris, presence of organisms, sediment substrate (i.e., sand, silt, gravel), signs of scouring, water depth, outfalls, and other features can be helpful when interpreting results of site-specific studies. Therefore, all observations should be documented in a field log book and photographs should be taken of the sediment and sample locations. Any field variances of the SAP should be clearly documented in the field log book. These observations should be presented in a summary table to aid the reviewer of the BERA (Table 21-14).
| Table 21-14. Example of Qualitative Field Notes | ||||||
| Station | Redox Discontinuity (cm) | Sediment Description | Biota Present | Other Comments/Notes | Photos | |
| SD01 | < 1 | black color, silt/clay with some sand | worm burrows, iron secretions | Collected samples in the mudflat located on the peninsula on the side facing the bridge. | 2 | |
| SD02 | no redox | red-brownish color sand with some silt | none observed | 1 | ||
| SD03 | 3 | medium brown sand with medium grey silt below | worm burrows | Moved 14 feet toward water because riprap was present at proposed sample location. | 1 | |
| SD04 | < 1 | black silt | one mussel shell (open) | Collected sample 30 feet south of 2nd wooden pier. | 1 | |
| SD05 | 1 to 4 | brown sand at surface, brown/dark grey to black silt below | limu, eelgrass, some live gastropods | 3 | ||
| Redox Discontinuity – Depth at which the color changes from brown to gray/black | ||||||
21.6.3.2 ANALYTICAL RESULTS
Data that will be used in a risk assessment should undergo a Stage 4 data validation in accordance with the USEPA National Functional Guidelines to ensure that the data are of good quality and are legally defensible. Methods for validating the data should be given briefly in the BERA WP/SAP and explained fully in the QAPP, along with the criteria for determining the acceptability of the data. Guidance on data validation is available from USEPA through the Contract Laboratory Program National Functional Guidelines for Data Review (USEPA 2018).
Data packages should also be reviewed to determine whether any data should be rejected and whether any data qualifiers assigned during the validation process affect the usability of the data as defined in the QAPP. The validated analytical data packages should contain a summary of all data qualifier flags and their explanations.
Analytical results for all media should be presented in summary statistics tables including the following information: chemical name and CAS number, number of samples analyzed, maximum and minimum detected concentrations, data qualifiers, range of detection limits, and frequency of detection. When samples sizes are large enough (n>10), estimates of the mean such as the 95 percent upper confidence limit on the mean concentration (UCL95) may be used to represent the exposure point concentration in the DU. See TGM Section 4 for more information on calculating UCL95. When appropriate, separate tables that show results only for chemicals detected in at least one sample may be presented to focus the BERA. However, whenever a result is listed as “not detected,” the sample-specific detection limit must be given in the table.
The sample-specific detection limits reported by the laboratory should be reviewed prior to using the data in the BERA. If the laboratory was not able to meet the detection limits presented in the WP/SAP, the data may not be useable for the BERA. Regardless of the format of tables chosen by the risk assessor, all data for all analyzed parameters, including parameters not detected in any sample, must be included as appendices to the BERA.
Pay close attention to concentration units (e.g., µg/kg, mg/kg) in all tables and throughout the text. Laboratory results, regulatory criteria, and published literature may use different units. It is the risk assessor’s responsibility to convert all units to a uniform standard so that meaningful comparisons can be made. Many components of the BERA incorporate ratios (such as hazard quotients and bioaccumulation factors) that are rendered meaningless when units are not consistent. Likewise, double-check that the dry-weight or wet-weight concentrations are properly represented. In peer-reviewed publications, this detail may appear only in a table or figure legend rather than stated explicitly in the text. When in doubt, contact the HEER Office for assistance.
21.6.3.3 TOXICITY AND BIOACCUMULATION TESTS
The BERA should refer to the description of toxicity and bioaccumulation tests proposed in the WP/SAP and explain any variances to the proposed procedures. When results of the laboratory toxicity tests are presented, the reasons for variances and potential effects of results should be explained. For example, the laboratory technician may have decided to aerate the samples because the dissolved oxygen level decreased below a certain threshold.
The full laboratory toxicity test report should be included as an appendix to the BERA and the results summarized in the BERA. The format of results may vary with the type of test; Table 21-15 is provided as an example only. Any potentially confounding factors, such as high ammonia or low dissolved oxygen, should be discussed in the text. The laboratory control sample results should be evaluated to determine whether the test met acceptability criteria.
| Table 21-15. Summary of Leptocheirus plumulosus Toxicity Test Results | |||||
| Sample Number | Mean Survival (%) | Mean Dry Weight (mg/organism) | Mean Overall Juvenile Production (juveniles/ amphipod) | Mean Juvenile Production per Surviving Female (juveniles/female amphipod) | |
| Lab Control Sample | 85 | 1.47 | 6 | 13 | |
| Reference Samples | |||||
| RF-SD01 | 83 | 1.40 | 7 | 13 | |
| RF-SD02 | 84 | 1.48 | 6 | 14 | |
| RF-SD03 | 80 | 1.52 | 6 | 12 | |
| Site Samples | |||||
| SD101 | 63 | 0.99 | 6 | 12 | |
| SD102 | 77 | 1.57 | 5 | 17 | |
| SD103 | 81 | 1.27 | 5 | 9 | |
| SD104 | 53 | 1.30 | 7 | 13 | |
| SD105 | 71 | 1.55 | 9 | 17 | |
Several methods can be used to evaluate toxicity test results. The three most common endpoints for sediment toxicity testing include (1) mortality as measured by survival of the amphipods; (2) growth as measured by weight and biomass; and (3) reproduction as measured by overall juvenile production and juvenile production per surviving female. The BERA WP/SAP should provide details on how results will be interpreted for any endpoints other than these.
Site samples are identified as toxic relative to the reference samples using a statistical test. The laboratory control samples are included simply to determine whether the test organisms were healthy; laboratory controls are not used to evaluate site-specific toxicity. Methods to interpret toxicity test results should have been specified in the BERA WP/SAP and discussed with the HEER Office. (Guidance on statistical tests appropriate for analyzing toxicity test results is under development and will be added to this TGM when available.)
Laboratory bioaccumulation studies should be treated in the same way as toxicity studies, with the added component of final tissue concentrations. As discussed previously, tissue concentrations should be provided in dry weight, along with percent moisture and percent lipid results. Tissue results from laboratory bioaccumulation tests should be presented in the same way as field-collected tissues, with the additional component of calculated BSAFs, if warranted.
Risks to Receptors from Food Chain Exposure
Tissue concentrations are used in food chain models to estimate daily doses to consumers, as described in Subsection 21.3.3. While the SLERA intentionally biased the estimated daily dose high using conservative exposure parameters, the average dose is used in the BERA to represent a more realistic exposure scenario. The focus of the BERA is risk to populations of receptors, not to individual organisms. Therefore, average exposure assumptions are used. For example, the estimated daily dose in the BERA should incorporate the components below:
- Mean chemical concentrations in sediment and food;
- Mean ingestion rates for sediment and food;
- Mean body weight;
- Appropriate site use factor; and
- Most sensitive life stage present at the site.
In the SLERA, concentrations in food are estimated from concentrations in sediment using BSAFs as described in Appendix 21-E. However, if site-specific tissue samples were analyzed in the BERA, those concentrations should be substituted in the dose equation. Alternatively, if site-specific BSAFs are determined in the BERA, they should be used instead of BSAFs from the literature to estimate tissue concentrations at the site.
21.6.4 RISK CHARACTERIZATION
The risk characterization subsection of the BERA is where all available data are evaluated holistically to determine whether the site poses unacceptable risk to any of the assessment endpoints. The risk characterization should present both quantitative and qualitative characterizations of risk, to the extent supported by available data. As described in Step 3a (Task 6), the risk characterization focused on interpreting exposure and effects data within the context of other site-specific information. Risk characterization in the BERA is similar, in that it synthesizes all available data and various sources of uncertainty, while acknowledging data gaps that may limit conclusions.
When multiple measures of effect are available for a single assessment endpoint, then a weight-of-evidence approach should be used to interpret the implications of different datasets. For example, as discussed in Subsection 21.6.2.5, biological surveys are often collected as part of a sediment triad approach where three lines of evidence (sediment chemistry, sediment toxicity test data, and benthic community data) are evaluated to as part of an overall investigation of the benthic community. This can be done by assigning each line of evidence a score and associated weighting factors.
The risk assessment results can be presented graphically to highlight locations where chemical concentrations exceed toxicity screening levels that were identified in the BERA WP/SAP. Maps and graphs may be used to illustrate spatial distribution of risk using various measures. The HEER Office can offer examples of effective data presentation methods, as needed.
