Section 4 Site Investigation Design and Implementation: Appendices
NOTE: This page is under construction. See the PDF downloads for Section 4
Interim Final – June 2024
Foreword to Sections 3 and 4
Appendix A Fact Sheets
Fact Sheet #1: Use of DU-MIS Sampling Methods for Risk-Based Investigation of Contaminated Soil and Sediment
Fact Sheet #2: Sampling Plan Essentials for Responsible Parties
Appendix B Conceptual Site Models
B.1 Summarize Known Site Conditions
B.2 Screen for Potential Environmental Hazards
B.3 Default Conceptual Site Models
B.4 Advanced Site Conceptual Models
B.5 Maintaining and Updating the Conceptual Site Model
Appendix C Example Decision Unit Designation Schemes
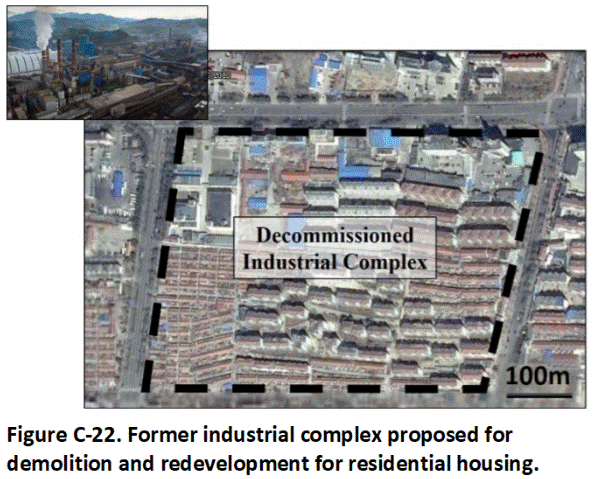
C.1 Characterization of Very Large Areas for Redevelopment
C.2 Commercial and Industrial Sites
C.3 Single-Family Homes
C.4 High-Density Housing
C.5 Schools
C.6 Large Single-Use Areas
C.7 Very Small Areas
C.8 Subsurface Decision Units
C.9 Stockpiles
C.10 Building Demolition
C.11 Large Industrial Complexes
C.12 Investigation of Petroleum Releases
C.13 Gas Station Closure
C.14 Sediment Decision Units
Appendix D Sampling Theory and Multi Increment Sample Collection
D.1 Theory of Sampling
D.2 Application to Environmental Sampling
Appendix E Use and Misuse of Discrete Sample Data
E.1 Background and Key Assumptions
E.2 Field Study of Discrete Sample Data Reliability
E.3 Implications of for Reliance on Discrete Sample Data
E.4 Contamination Zones
E.5 Use of Discrete Sample Data for Preliminary Screening
Appendix F Collection of Surface Soil Samples
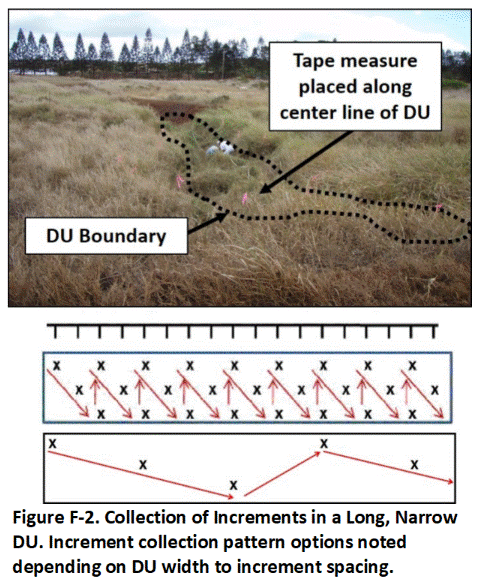
F.1 Locating DU Increment Collection Points
F.2 Increment and Bulk Sample Collection
F.3 Sample Collection Tools and Field Methods
Appendix G Collection of Subsurface Soil Samples
G.1 Exploratory Pits, Trenches and Borings
G.2 Direct-Push Technologies
G.3 Borehole Core Increment Subsamples
G.4 Pit or Trench Sample Collection
G.5 Hollow Stem Augers
G.6 Rotary Drills
Appendix H Collection of Excavation and Stockpile Samples
H.1 Excavations
H.2 Stockpiles
Appendix I Sample Collection for Volatile Contaminants
I.1 Overview
I.2 Increment Collection and Sample Preparation
I.3 Subsurface DU Layers
I.4 Sample Shipment
I.5 Alternatives to Methanol Preservation
Appendix J Collection of Sediment Samples
J.1 General
J.2 Small, Shallow Water Bodies
J.3 Large, Shallow Water Bodies
J.4 Deep Water
J.5 Diver Assisted Sample Collection
J.6 Other Devices
Appendix K Laboratory Processing of Multi Increment Samples
K.1 Introduction
K.2 Sample Processing
K.3 Subsample Collection
K.4 Analytical Subsample Mass
K.5 Particle Size Reduction
K.6 Semi-volatile and Unstable Chemicals
K.7 Testing of Samples for Contaminant Bioaccessibility
K.8 Soil Leaching Tests
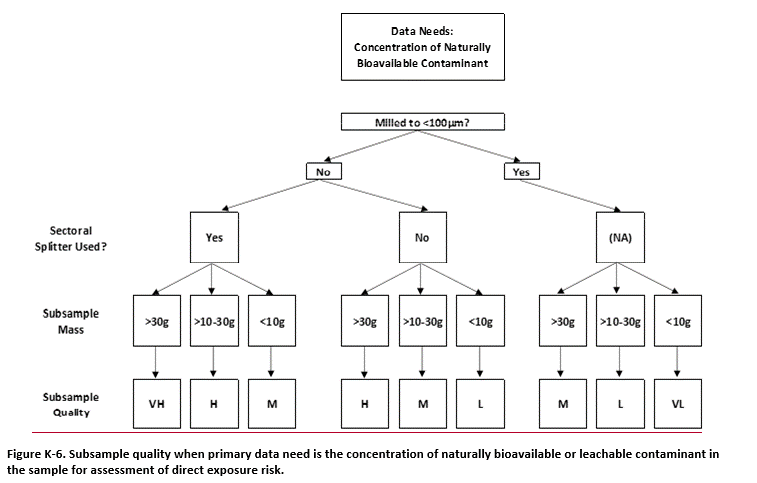
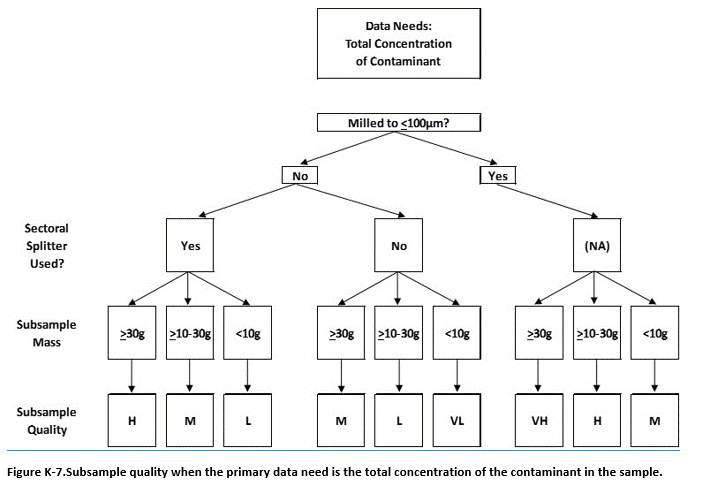
K.9 Assessment of Overall Subsample Data Quality
Appendix L Collection and Evaluation of Replicate Sample Data
L.1 Collection of Field Sample Replicates
L.2 Collection of Laboratory Subsample Replicates
L.3 Evaluation of Data Usability
Appendix M General Field Operations
M.1 Field Documentation
M.2 Equipment Preparation/Decontamination
M.3 Investigation Derived Waste
M.4 Field Work Completion
Appendix N Common Investigation Errors and Problems
N.1 Inappropriately Sized DUs
N.2 Data Gaps Between Surface DUs and Subsurface DU Layers
N.3 Inadequate Number of Increments and Bulk Sample Mass
N.4 Improper Increment Spacing
N.5 Improper Increment Shape
N.6 Misuse of Co-located Discrete Samples and Increment Splits
N.7 Inadequate Laboratory Processing
N.8 Inadequate Subsample Mass for Analysis
N.9 Lack of Field Replicate Sample Data
N.10 Reversion to Discrete Sampling
Appendix O DU-MIS Investigations Under TSCA
Appendix P Clean Fill Guidance
Appendix Q References
FACT SHEET #1: Use of DU-MIS Sampling Methods for Risk-Based Investigation of Contaminated Soil and Sediment
This fact sheet provides government regulators, consultants, property owners and other interested parties with a brief overview of Decision Unit and Multi Increment® Sample (DU-MIS) investigation methods for contaminated soil (Multi Increment® is registered trademarked of EnviroStat, Inc.). This fact sheet is an accompaniment to the “Site Investigation Design and Implementation” sections of the State of Hawai´i, Department of Health Technical Guidance Manual (Sections 3 and 4; HIDOH 2023). The examples presented focus on soil, but similar approaches are applied to testing of sediment and other particulate media.
What is DU-MIS?
 Decision Unit and Multi Increment Sample investigation methods are a risk-based strategy to test soil and determine if contamination poses a potential threat to human health and the environment. The methods were specifically designed to address concerns related to the unreliability of “discrete” sampling methods widely utilized in the environmental industry. The DU-MIS approach can require additional time and effort at the beginning of a project but will ultimately help to:
Decision Unit and Multi Increment Sample investigation methods are a risk-based strategy to test soil and determine if contamination poses a potential threat to human health and the environment. The methods were specifically designed to address concerns related to the unreliability of “discrete” sampling methods widely utilized in the environmental industry. The DU-MIS approach can require additional time and effort at the beginning of a project but will ultimately help to:
- Provide a clear endpoint to an environmental investigation;
- Reduce total project duration and cost;
- Ensure sample data collected are reliable and reproducible;
- Provide a higher degree of confidence that potential risks have been identified and addressed;
- Provide confidence that cleanup actions are only conducted where warranted; and
- Avoid unanticipated delays or even abandonment of projects due to time and cost overruns and lack of a clear endpoint.
These methods apply to nonvolatile and volatile contaminants as well as surface and subsurface soils. Similar sampling methods have been used for decades by the mineral exploration and agriculture industries but are relatively new to the environmental industry, where the error in the representativeness of sample data is less evident.
How is DU-MIS Implemented in the Field?
DU-MIS investigation methods are carried out in a methodical manner that helps increase confidence in the representativeness of the data collected and decisions made based on the data. This step-by-step process, which includes inspecting the site, talking to people familiar with the site history and compiling existing data, is referred to as “Systematic Planning.” A nine-step process is described in the DU-MIS guidance associated with this fact sheet. Below is a condensed five-step process for ease of explanation.
 Step 1: Review the Site History
Step 1: Review the Site History
The first step in a “risk-based” investigation is to gain a thorough understanding of the site before samples are even collected. The information is summarized in a preliminary “Conceptual Site Model (CSM).” The CSM is then used to design the site investigation.
Step 2: Designate Decision Units for Sample Collection
The second step is to designate well-thought-out areas of the site, referred to as “Decision Units (DUs),” to be individually tested for contamination. A DU can be thought of as an area or more specifically volume of soil or sediment that would ideally be sent to a laboratory for testing as a single sample. Each DU is designated to address a specific site investigation question regarding the assessment of risk to human health and the environment and/or the optimization of potential remedial actions.
Decision Units are set to the size needed to address the questions asked and the objectives of the investigation. A “Decision Statement” is assigned to each DU. This statement specifies the action to be taken when sample data are received and is prepared prior to the collection of a sample. This provides a clear pathway forward for subsequent stages of the investigation once sample data are obtained and helps expedite the overall completion of the project.
Risk-based DUs should be selected based on site history and current potential exposure pathways. Exposure Area DUs include unpaved areas where children and adults frequently play or work, such as playgrounds, schoolyards, gardens, open areas of commercial and industrial sites and exposed soil at construction sites. These are a very common components of human health risk assessments. The exact size of an Exposure Area DU is site-specific but normally ranges from a few thousand to a few tens of thousands of square feet in area and from one hundred to several hundred cubic yards of soil in volume with the upper two to six inches tested.
 Areas of known or suspected heavily contaminated soil that are almost certain to pose a risk if exposed at the surface are designated for separate testing to optimize anticipated remediation. These are referred to as Source Area (or Spill Area) DUs. Source Area DUs are surrounded by anticipated clean Boundary DUs to isolate areas of heavy contamination to the extent practicable and cost-beneficial in terms of anticipated remediation needs. Successful remediation of contamination can be verified by designation and testing of Exposure Areas DUs in the same locations.
Areas of known or suspected heavily contaminated soil that are almost certain to pose a risk if exposed at the surface are designated for separate testing to optimize anticipated remediation. These are referred to as Source Area (or Spill Area) DUs. Source Area DUs are surrounded by anticipated clean Boundary DUs to isolate areas of heavy contamination to the extent practicable and cost-beneficial in terms of anticipated remediation needs. Successful remediation of contamination can be verified by designation and testing of Exposure Areas DUs in the same locations.
DUs are designated to characterize both surface soil and, as needed, subsurface soil. Subsurface soil is characterized in terms of stacked DU Layers. Suspect layers of subsurface soil, identified by site history, initial surface soil data or other observations, should be designated for separate testing to bound the vertical extent of the contamination. Designation of subsurface DUs is normally done at a scale that will assist in optimization of potential remediation. Testing and documentation of subsurface contamination might also be performed for long-term management purposes to avoid potential excavation of the material in the future and inadvertent reuse on the surface.
The size and number of DUs designated to characterize a site reflects the “resolution” of the investigation necessary to answer the questions being asked, much like the pixels of a digital photograph. Five to ten DUs are normally adequate to characterize a simple site. Twenty or more surface and subsurface DUs might be required to characterize a complex site.
Step 3: Collect a Representative Sample from Each DU Area
 Decisions regarding both risk and remediation are always based on the true concentration of the contaminant for the DU volume of soil as a whole. This is often referred to as the “mean” concentration in environmental documents. All data for particulate matter necessarily represents a mean of the collective group of particles tested, however, and use of this term is unnecessary.
Decisions regarding both risk and remediation are always based on the true concentration of the contaminant for the DU volume of soil as a whole. This is often referred to as the “mean” concentration in environmental documents. All data for particulate matter necessarily represents a mean of the collective group of particles tested, however, and use of this term is unnecessary.
Under ideal circumstances the entire DU volume of soil or sediment would be excavated and submitted to the laboratory for testing as a single unit. This is not practical under most circumstances and a representative sample of the material must be collected instead. The science and statistics behind the collection of a representative sample of soil is complex and involves the need to address both variability between individual particles (“compositional heterogeneity”) and variability within the targeted DU (“distributional heterogeneity”). The procedure to collect a sample in the field is, however, relatively straightforward.
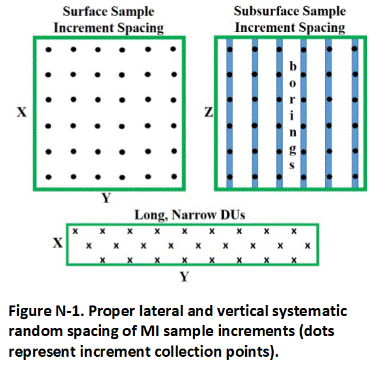
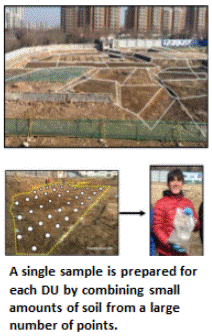
A single sample is prepared for each DU by collecting and combining small, core-shaped masses of soil from a large number of points within the targeted area. The soil from each point is referred to as an “increment” and the combined increments are referred to as a “Multi Increment (MI)” sample. A minimum of 30 to 75+ increments with a combined mass of 1 to 3 kilogram (kg) is normally required to prepare a reliably representative sample that is to be tested for non-volatile chemicals.
A default of 50 increments per sample is recommended. This will provide reliably representative sample data under most site scenarios based on past field experience and comparison of replicate sample data. Fewer increments might be acceptable for testing of liquid releases (anticipated lower heterogeneity). A larger number of increments is required for contaminants present in the soil as clumps or chips (anticipated higher heterogeneity).
This sample collection method provides a high degree of confidence that the resulting data will be representative of the targeted DU pertinent to the investigation questions being asked. For added certainty, two additional, independent samples are collected from at least one of the DUs. These are referred to as “replicate” samples and are used to evaluate the overall precision of the sampling method and reproducibility of the sample data. Although this adds to the cost of the investigation, replicate samples are critical to demonstrate the reproducibility and defensibility of the data and should always be included in an investigation. Laboratories also collect replicate subsamples for testing in order to document the reproducibility of sample data. Other types of replicate samples, including testing of replicate subsamples from cores, can add to confidence in the quality and representativeness of the data.
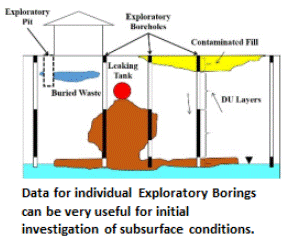 Direct-push rigs or excavators can be used to collect increments and prepare MI samples from subsurface DU Layers. If the collection of 50-increment MI samples is not possible due to drilling obstructions or other challenges, then this should be discussed with the overseeing regulatory agency and the limitations of the resulting data should be noted. In some cases, it might be necessary to make decisions based on data for individual borings. The boring itself then becomes the DU, referred to as an “Exploratory Boring DU.” Each targeted interval within the boring becomes an individual DU Layer. If the targeted interval of core is too large to submit to the laboratory for testing, then an MI sample is collected. Data from Exploratory Borings can be useful for a very general estimation of the extent and magnitude of subsurface contamination, especially in the case of subsurface petroleum and solvent releases. Be aware however, that there is a risk of “false negative” results when using this approach and underestimation of the magnitude and extent of contamination. Full DU-MIS testing of the soil is required for confirmation.
Direct-push rigs or excavators can be used to collect increments and prepare MI samples from subsurface DU Layers. If the collection of 50-increment MI samples is not possible due to drilling obstructions or other challenges, then this should be discussed with the overseeing regulatory agency and the limitations of the resulting data should be noted. In some cases, it might be necessary to make decisions based on data for individual borings. The boring itself then becomes the DU, referred to as an “Exploratory Boring DU.” Each targeted interval within the boring becomes an individual DU Layer. If the targeted interval of core is too large to submit to the laboratory for testing, then an MI sample is collected. Data from Exploratory Borings can be useful for a very general estimation of the extent and magnitude of subsurface contamination, especially in the case of subsurface petroleum and solvent releases. Be aware however, that there is a risk of “false negative” results when using this approach and underestimation of the magnitude and extent of contamination. Full DU-MIS testing of the soil is required for confirmation.
Sample collection methods for volatile chemicals require that separate increments are combined in a bottle containing a pre-measured volume of methanol. Further details on sample collection methods for volatile chemicals are discussed in Appendix I.
Step 4: Sample Processing and Analysis
Contact the laboratory during the planning phase to ensure that they are experienced in processing and testing of MI samples. Ensure also that the laboratory can achieve the desired reporting limits and data quality objectives. Select analyses that achieve the desired risk concerns and goals. Avoid testing for unneeded unknowns to keep costs under control.

Once collected, the sample is sent to a laboratory for processing and testing. The laboratory will not be able to test the entire 1 to 3 kg sample. Strict protocols must be followed in order to collect a representative subsample for testing. The bulk field sample is normally air dried for 24 to 48 hours and then passed through a sieve to remove large rocks and other debris and isolate the target particle size (e.g., <2 millimeters). A sectoral splitter is then used to collect a representative subsample (third photo in the figure above). Although more prone to error, the sample can also be spread into a thin layer and a subsample is then manually collected from 30 or more points (increments), similar to how the sample was collected in the field.
These steps help ensure that the laboratory data are representative of the sample and that the sample submitted is representative of the targeted DU. The laboratory is required to test two additional replicate subsamples (laboratory “triplicates”) collected from 10% of the samples submitted to test the precision and reliability of the subsampling method. These data are again critical to demonstrate the defensibility of the data and should always be included.
Step 5: Data Review and Decision Making
When the laboratory data are received, a review of the overall reliability of the data is made based on field and laboratory replicate samples and other quality control measures. If the replicate data are very different and the problem is determined to be at the laboratory, then retesting of the samples might be required. If the problem is determined to be related to the method used to collect the samples in the field, then the sampling process will be reviewed and the collection of new samples might be required. Field error is often due to the presence of previously unidentified, highly heterogeneous source areas within the initially targeted DU. Error associated with sample collection and laboratory testing decreases as experience is gained.
Once the data are determined to be usable, the data for each DU can be directly compared to risk-based screening levels applicable to the investigation question(s) of interest and decisions can be made on the need for cleanup or other soil management actions. The need to collect additional samples should be minimal, assuming that DUs were appropriately designated at the beginning of the project and DU questions and decision statements were properly prepared ahead of time.
Why are DU-MIS Sampling Methods Necessary?
Guidance for the investigation of contaminated sites published by the USEPA in the 1980s focused on the collection and testing of individual, small masses of soil from single points referred to as “discrete” samples. The authors noted that this method would only be reliable if the concentration of a contaminant in soil was very uniform both within a sample and between closely spaced samples.
Scientists and field workers began to warn in the early 1990s that this was not the case. Data for co-located samples often varied widely and randomly, as did data for duplicate subsamples tested by the laboratory. This caused confusion in the field regarding the extent of contamination above levels of potential concern and in the assessment of risk. The need to repeatedly remobilize field teams for sample collection and the discovery of additional contamination after remediation was thought to be completed, caused some projects to be delayed for years and in some cases to be abandoned due to the lack of a clear endpoint.
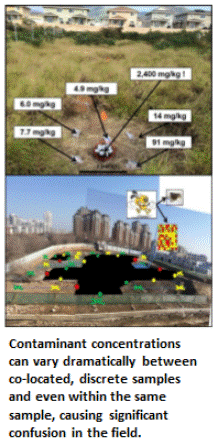 A thorough field study of the reliability of discrete sample data for testing of environmental sites was, surprisingly, not carried out until 2015 – thirty years after the first USEPA site investigation guidance was published (Brewer et al. 2017a and b). The field study verified contaminant concentrations can vary dramatically and randomly between samples collected just a few inches from each other and even within an individual sample. Statistical analysis of independent (replicate) sets of discrete samples can predict very different mean contaminant concentrations and associated risks for targeted exposure areas. These factors are the primary cause of project completion delays, cost overruns and the later discovery of significant contamination in areas previously declared to be “clean.”
A thorough field study of the reliability of discrete sample data for testing of environmental sites was, surprisingly, not carried out until 2015 – thirty years after the first USEPA site investigation guidance was published (Brewer et al. 2017a and b). The field study verified contaminant concentrations can vary dramatically and randomly between samples collected just a few inches from each other and even within an individual sample. Statistical analysis of independent (replicate) sets of discrete samples can predict very different mean contaminant concentrations and associated risks for targeted exposure areas. These factors are the primary cause of project completion delays, cost overruns and the later discovery of significant contamination in areas previously declared to be “clean.”
The mineral exploration and agricultural industries recognized the same problems many years ago. Gold exploration companies often went bankrupt when the amount of gold initially estimated to be presented in stockpiles of crushed ore, based on traditional sampling methods accepted at the time, proved dramatically different from the mass of gold ultimately extracted from the ore after selling it to a processor. Farmers realized the unreliability of discrete sample data very quickly, as crop yields failed to meet expectations or large sums of money were unnecessarily spent on fertilizer or other field amendments.
The result was the development of the Theory of Sampling by Pierre Gy in the 1950s. The Theory of Sampling serves as the basis of the DU-MIS methods described in this fact sheet. Errors in sample data and decision making are less obvious in the environmental industry, but DU-MIS methods are continually improved to make the investigation, assessment and remediation of contaminated soil as efficient and reliable as possible.
Where can I get more information?
Brewer, R., Peard, J. and M. Heskett. 2017. A critical review of discrete soil sample reliability, (two parts): Soil and Sediment Contamination, Vol. 26 (1).
HIDOH, 2023, Technical Guidance Manual, Sections 3&4 Site Investigation Design and Implementation: State of Hawai´i, Department of Health, July 2023 (and updates).
FACT SHEET #2: Sampling Plan Essentials for Responsible Parties
Samples of soil, soil vapors, surface water are sometimes required as part of a property transaction or to cleanup known or suspected contamination. The resulting sample data are normally compared published “action levels” or “screening levels” to determine if contamination that mire require cleanup is present.
A Sampling and Analysis Plan (SAP) is prepared by an environmental consultant and tailored specifically for your project. It is unique to your property and can be customized to your timeline and budget. An SAP is only as good as the objectives it’s based on, however. Review and approval of the SAP by the overseeing regulatory agency is sometimes required. In other cases, the agency might only review the report that summarizes the results of the sampling. It is important to have a clear idea of your regulatory and other needs before samples are collected. If your plans or objectives change, then your consultant will need to update your SAP.
 Option 1: Quick and Cost-Effective Screening
Option 1: Quick and Cost-Effective Screening
- Property divided into a small number of “Decision Units (DUs)” for sample collection based on past and current use.
- Relatively cheap and fast – ideal for initial screening.
- Can provide advanced warning for unexpected cleanup costs.
- Adequate to assess direct-exposure risk for commercial land use.
- Easily adjusted to meet excess soil reuse or disposal data needs.
Option 2: Sampling for Minimum Property Use Restrictions
- Property divided into a larger number of smaller DUs.
- Improved resolution allows for potential unrestricted property use and offsite reuse or disposal of excess soil.
- Provides quantitative data to support property valuation and transaction decisions.
- Results can be used to estimate cleanup cost and guide initial actions.
Option 3: Sampling for Cleanup Savings
- All localized areas of known or suspected contamination designated as separate DUs for sample collection.
- Isolates areas of contamination for preparation of remedial plan and optimization of cleanup time and cost.
- Minimizes the need for additional mobilization to collect samples.
- Helps control quantities of soil requiring treatment or disposal.
Tips for Adapting Your Sampling Plan
- Large Decision Units (DUs) are usually only used for screening purposes. Smaller DU areas are normally needed to optimize cleanup and control costs for soil treatment, disposal and re-use.
- While your consultant can adapt your SAP to meet your specific needs (see table below), if large DUs are initially designated for testing and contamination is discovered, then smaller DUs and additional rounds of sampling using smaller DUs might be necessary to optimize cleanup costs.
- It is always best to get started early and to get input from the overseeing regulatory agency as you go. Be aware, however, that staffing limitations and time constraints might preclude formal review and approval of an SAP. It is therefore important to work with your consultant to ensure that the data collected will meet the needs of the regulatory agency for review and approval of investigation and cleanup of the property.
| Initial Investigation | Later Cleanup Actions | ||
| Action | Save Money | Save Time | Increased Likelihood of Additional Sampling? |
| 1Optimize DU number and size for potential cleanup | No | No | No |
| 2Increase DU size & decrease number of DUs | Yes | Yes | Yes |
| 2Reduce number of increments, particularly for deep investigations that require drilling | Yes | Yes | Yes |
| 2Stage investigation over multiple mobilizations | Yes | No | No |
| 3Hold deeper samples pending initial sample results | Yes | No | No |
| 3Hold contingent analyses pending initial sample results | Yes | No | No |
| 1Option 3 above. Potential to save overall time and cost if contamination known or suspected due to reduced cleanup costs/mobilizations; costs more/takes longer up front. | |||
| 2Options 1 & 2 above. Potential to spread out and/or save on initial investigation costs; may end up costing more/taking longer overall if unanticipated contamination identified and cleanup required. | |||
| 3Additional approaches can be taken by experienced consultants to reduce the cost of investigation and cleanup actions, although more time might be required to complete the project. | |||
APPENDIX B. CONCEPTUAL SITE MODELS
The Conceptual Site Model (CSM) prepared during the first step of the systematic planning is a comprehensive representation of site environmental conditions with respect to recognized or potential environmental hazards. The CSM is continuously updated as the site investigation progresses, and the site conditions are better understood.
A basic understanding of contaminant migration pathways and exposure pathways is necessary to formulate a CSM and to guide site investigation and response actions, including preparation of remedial actions and/or long-term management plans. Preparation and submittal of a formal, detailed CSM, however, is generally only required at sites where significant contamination exists and cleanup activities are anticipated to take more than a year to complete.
Return to the Top of the Page
B.1 SUMMARIZE KNOWN SITE CONDITIONS
The first step in the preparation of a CSM is to summarize current site conditions. At the most basic level, this includes a summary of the known or suspected extent and magnitude of soil and groundwater contamination. In addition, site conditions such as land use, groundwater use, potential onsite and offsite receptors, exposure or isolation of contaminated soil, etc., are identified, as are specific environmental hazards that might be posed by the identified contamination.
Return to the Top of the Page
B.2 SCREEN FOR POTENTIAL ENVIRONMENTAL HAZARDS
A basic understanding of potential environmental hazards in terms of the environmental fate and transport of contaminants of potential concern (COPCs) targeted for a site is important for development of a CSM and subsequent stages of an investigation. As discussed in Section 3.2.2 and in Appendix C, the designation of DUs is intricately tied to the type of environmental hazard(s) posed by the COPC.
Common environmental hazards associated with contaminated soil and groundwater include as follows (Figure B-1):
-
- Long-term chronic risk to humans caused by long-term direct exposure to contaminants in soil, sediment, water or air.
- Short-term acute risk to humans caused by strong vapor emissions from temporary exposure of heavily contaminated soil or groundwater.
- Chronic and acute risk posed to terrestrial and aquatic ecological receptors.
- Intrusion of vapors from soil or groundwater into overlying buildings.
- Leaching of contaminants from soil and contamination of groundwater or surface water; and
- Short-term risk of fire, strong vapor emissions, odors, sheens in stormwater runoff, fouling of construction equipment and other gross contamination problems related to widespread petroleum contamination.
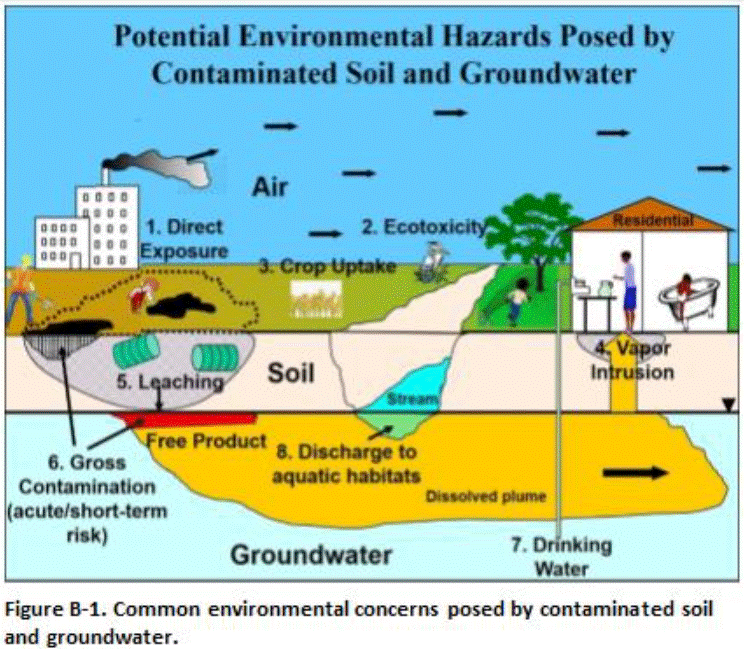
Potential concerns are identified by comparison of site data to pertinent screening criteria or the use of models to estimated risk based on predicted contaminant fate and transport and receptor exposure scenarios.
Environmental risk is always assessed based on the true (“mean”) concentration of the targeted contaminant for a designated area and volume and soil as a whole, rather than at discrete points (USEPA 1987, 1988c, 1989b, c, e, f, 1991g, 1992c, 2014f)
Return to the Top of the Page
B.3 DEFAULT CONCEPTUAL SITE MODELS
Default CSMs can provide a useful starting point for the preparation of site-specific CSMs and might be required by some regulatory agencies. It is important that the CSM takes into consideration all potential environmental concerns relevant to the subject site. Site-specific factors such as current and anticipated land use, the utility of underlying groundwater and the potential for contaminated groundwater to discharge into a nearby aquatic habitat must also be taken into consideration. The rationale for excluding specific concerns should be clearly discussed in the report.
The default CSMs can also be depicted in a more classical “risk assessment” format, as presented in Figure B-2. The hypothetical site is contaminated with petroleum from leaking aboveground storage tanks (ASTs), underground storage tanks (USTs), pipelines, drum storage areas and disposal areas. As a default, the site is assumed to overlay groundwater that is a source of drinking water and be adjacent to a surface water body with impacted soil exposed at surface.
Figure B-2. Default Conceptual Site Model for a petroleum-contaminated site.
| Primary Sources | Primary Release Mechanism | Secondary Sources | 1Potential Environmental Hazards | 2Hazard Present Under Current or Future Site Conditions? | ||
| Current | Future | |||||
| ASTs, USTs, pipelines, drums, disposal areas, etc. | Spills, leaks, improper disposal | Soil | 3Risk to Human Health | Direct Exposure | YES | YES |
| Vapor Intrusion into Buildings | YES | YES | ||||
| 4Risk to Terrestrial Ecological Habitats | YES | YES | ||||
| 5Leaching | YES | YES | ||||
| 6Gross Contamination | YES | YES | ||||
| Groundwater | 7Risk to Human Health | Direct Exposure | YES | YES | ||
| Vapor Intrusion into Buildings | YES | YES | ||||
| 8Risk to Aquatic Ecological Habitats | YES | YES | ||||
| 9Gross Contamination | YES | YES | ||||
CSM assumptions:
- Example potential environmental hazards (modified on a site-by-site basis as appropriate).
- All listed hazards assumed present or potentially present and exposure pathways complete under current or future site conditions in the example.
- Human health hazards include direct exposure to contaminated soil or vapors and dust from soil in outdoor air as well as the intrusion of subsurface vapors into overlying buildings.
- Assumes a significant terrestrial, ecological habitat is impacted by the contamination with resulting toxicity to flora and fauna.
- Assumes potential leaching of contaminants from soil and impacts to underlying groundwater.
- Gross contamination hazards for soil include short-term, high-concentration emissions of hazardous vapors (e.g., during subsurface utility or construction work), potential fire and explosive hazards, sheens in stormwater runoff, fouling of equipment and related concerns.
- Human health hazards based on ingestion of contaminated groundwater as well as exposure via dermal absorption and vapor emissions during indoor use of water.
- Assumes discharge of contaminated groundwater into an aquatic habitat.
- Gross contamination hazards for groundwater include potential taste & odors concerns for drinking water, presence of free product, explosive hazards, odors, sheens, interference with construction work and other related concerns.
Return to the Top of the Page
B.4 ADVANCED SITE CONCEPTUAL MODELS
The template CSM presented in Figure B-2 is designed to be conservative and suitable for use in preliminary screening of a site. The CSM can be modified on a site-specific basis to more accurately reflect and assess potential environmental hazards under current and future site conditions.
Risk and cleanup needs are assessed based on the current and anticipated future use of the property. An assessment of requirements to remediate a site to unrestricted use (e.g., residential, schools, etc.) should always be included, even if the expected site use for the foreseeable future is only for commercial/industrial purposes. This will help ensure that formal restrictions are put in place to prevent inappropriate redevelopment of the property in the future as well as minimize unnecessary restrictions on the property should it already meet criteria for unrestricted reuse. The assessment might include a simple statement that additional investigation is required to clear the property for more sensitive uses. It could also include more detailed testing beyond what is called for to assess commercial/industrial use, such as the use of smaller DUs and comparison of data to both screening levels applicable to unrestricted land use and commercial/industrial land use.
Remediation to unrestricted use of the property is oftentimes only nominally higher in terms of cost than for commercial/industrial. In general, actions to address remediation of contaminated soil are financially manageable when the cost of the remediation is less than 10% of the total property redevelopment cost. Heavily contaminated sites are most effectively cleaned up by incorporating remedial actions into a large, redevelopment project.
Site-specific CSMs can be prepared by modifying the default CSMs to more closely evaluate potential environmental hazards under current and anticipated future site conditions. A more detailed CSM is generally warranted at sites where cleanup costs could be significant or at sites where long-term management of contaminated soil or groundwater will be required. A closer evaluation of current and future risks to human or ecological receptors will be particularly important. These types of CSMs will typically identify site-specific sources of contaminant releases, types of contaminated media, migration pathways, exposure pathways, and human and/or ecological receptors.Figure B-3 presents a more site-specific CSM for the same hypothetical commercial/industrial site contaminated with petroleum. The CSM includes the following site assumptions:
- Contamination is restricted to the site boundaries;
- Area of contaminated soil is paved;
- Underlying groundwater is not a current or potential source of drinking water; and
- Site is located more than 150 m (164 yd) from the nearest surface water body.
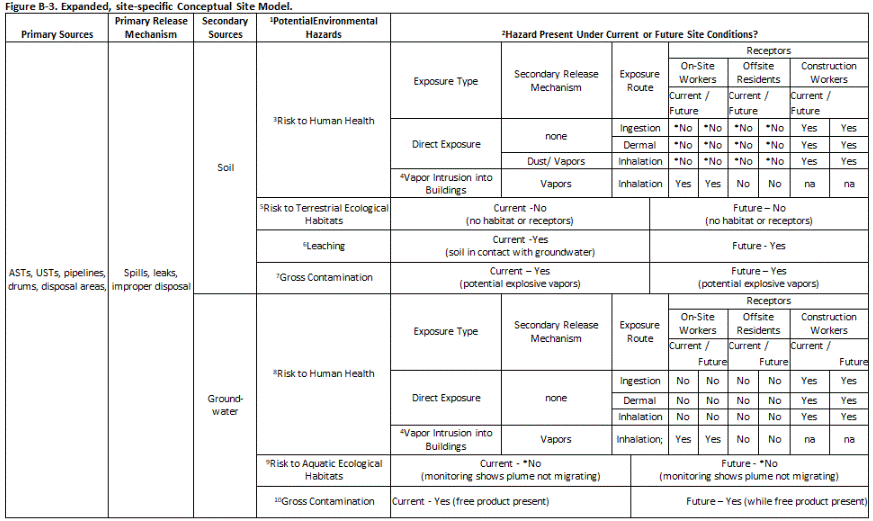 Figure B-3. Expanded, site-specific Conceptual Site Model.
Figure B-3. Expanded, site-specific Conceptual Site Model.
CSM Assumptions:
- Default environmental hazards to be initially evaluated.
- Hazard evaluation results based on assumption that contaminated soil is capped with pavement and contaminated groundwater is not migrating (e.g., naturally or via storm sewers, dewatering, etc.). *Long-term management of contamination must be addressed in a site-specific Environmental Hazard Management Plan in the absence of full cleanup.
- Exposure pathways for daily workers not complete *provided site remains paved. Potential exposure of construction workers during future subsurface activities.
- Recommend collection of soil gas data to further evaluate potential explosive hazards and vapor intrusion hazards.
- No significant terrestrial, ecological habitat located on site or threatened by contamination.
- Assumes contaminated soil is in direct contact with groundwater. Used to support collection of groundwater data for further evaluation.
- Recommend remediation of gross contamination at a minimum to reduce vapor concerns.
- Assumes groundwater is not used as a water supply and monitoring indicates that plume is not likely to migrate offsite under natural conditions.
- Threat to aquatic habitats assumed insignificant *provided plume is not allowed to migrate offsite. Contaminants screened using acute, aquatic toxicity action levels.
- Recommend removal of free product to extent practicable to reduce vapor concerns and continued source of contaminants to groundwater.
A “Yes” in a cell under “Receptors” indicates that the noted exposure route is complete or potentially complete. This is important information for development of short-term or long-term response actions to address human health or ecological risk concerns.
The CSM documents that the ingestion, dermal absorption and inhalation pathways for direct exposure to the contaminated soil are incomplete for daily on-site workers. Although the inhalation pathway could in theory still be complete, the presence of the pavement can reasonably be assumed to make this pathway insignificant. For construction workers, however, all direct-exposure pathways are considered complete because their work might involve removing pavement and disturbing contaminated soil.
The CSM indicates that the pathway for leaching of contaminants from soil and contamination of groundwater is complete, because contaminated soil is in direct contact with groundwater, even though the area is assumed to be capped with pavement. This is used to support the collection of groundwater data to evaluate impacts and potential concerns more directly. Removal of pavement could also exacerbate leaching and groundwater contamination due to infiltrating rain or irrigation water. This could require maintenance of an impermeable cap over the contaminated soil as part of a long-term management plan prepared for the site.
The CSM can be used to support a conclusion that contaminated soil and groundwater does not pose unacceptable environmental hazards under current site conditions. Depending on planned uses, active remediation to eliminate future environmental hazards under any potential land use condition could be recommended or required. If active remediation is not practicable due to current site use and conditions and/or financial constraints, the assumptions used in the CSM to support an absence of potential hazard under current site conditions can be used to develop a plan for long-term management of soil and groundwater. This is referred to as an “Environmental Hazard Management Plan (EHMP).” In the example, the EHMP would require that the area of contaminated soil remains capped, that a health and safety plan and soil and groundwater management measures be developed prior to any subsurface construction activities at the site, and that the need for long-term monitoring of groundwater be further evaluated.
Additional information on the development of CSMs is available in USEPA’s Guidance for Conducting Remedial Investigations and Feasibility Studies Under CERCLA (USEPA 1988b) and USEPA’s Data Quality Objectives Process for Hazardous Waste Site Investigations (USEPA 2000). Note that examples of CSMs in these guidance documents often focus on human health or ecological risk assessment concerns and might not consider other potential environmental hazards, including leaching and potential contamination of groundwater. The examples in the documents also might not reflect the transition to DU-MIS investigation methods.
Return to the Top of the Page
B.5 MAINTAINING AND UPDATING THE CONCEPTUAL SITE MODEL
The CSM should be maintained and updated as needed throughout the life of the site activities. As appropriate based on additional site information, refine the CSM to more accurately identify known or suspected sources of contamination, types and concentrations of contaminants detected at the site, potentially contaminated media, potential environmental hazards, potential exposure and migration pathways, potential human and environmental receptors, and related information.Information that should be used to maintain and continuously update the CSM includes (along with other relevant information):
- Identification of new or recently identified surface structures, subsurface utilities or other changes that might affect subsurface conditions, preferential pathways and risk to onsite or offsite receptors;
- Location of additional monitoring wells and past soil borings;
- Inclusion of additional soil, soil vapor or groundwater data;
- Updated soil, groundwater and soil vapor summary figures pertinent to the site with DU areas that exceed screening levels for specific environmental concern highlighted (referred to as Environmental Hazard Maps);
- Updated direction of groundwater flow, depth to groundwater, etc.;
- Updated cross sections that depict the site stratigraphy as well as the lateral and vertical extent of contamination; etc.
- Updated maps that depict DU areas that exceed screening levels or target risks for specific environmental concerns (referred to as Environmental Hazard Maps); and
- Consideration of data for advanced evaluations of specific environmental hazards (e.g., soil bioaccessibility data, soil vapor or indoor air data for assessment of vapor intrusions risks, etc.).
Significant changes to the CSM might necessitate updates to Decision Statements (Step 5 of Systematic Planning), the Sampling and Analysis Plan (Step 6 of Systematic Planning) and/or the plan for long-term management of contaminated soil and groundwater.
APPENDIX C. EXAMPLE DECISION UNIT DESIGNATION SCHEMES
This Section provides example Decision Units (DUs) for commercial/ industrial, residential, school, large area, subsurface, stockpile, and sediment sites. The examples reflect experience from actual projects but are not necessarily exact reproductions of any single project. The DUs depicted are for example only. Alternative DU configurations could be equally valid based on knowledge of the site, experience of the field team and the objectives of the investigation.
Return to the Top of the Page
C.1 Characterization of Very Large Areas for Redevelopment
Recommendations provided below apply to both residential and commercial/industrial redevelopment. Characterization of very large areas for redevelopment can be challenging. Such projects can cover hundreds or thousands of acres and include hundreds or thousands of individual residential lots. The primary environmental hazard is direct exposure of future residents and workers to residual pesticides, or other contaminants such as metals in the soil. Localized contamination of highly mobile chemicals (e.g. explosives residues) can also pose potential leaching threats to groundwater that might be used to serve the redevelopment in the future.
Table C-1. Recommendations for Investigation of Large Areas
| Project Classification | Area | Recommendations |
| Category 1 | <59 Acres |
|
| Category 2 | >59 to <118 Acres |
|
| Category 3 | >118 to <590 Acres |
|
| Category 4 | >590 Acres |
|
A default Exposure Area DU size of one acre is generally acceptable for characterization of large areas where no localized areas of potentially heavy contamination are identified as part of a thorough Phase I Environmental Site Assessment (ESA), for example suspect Spill Areas. Variability of mean contaminant concentrations within this default DU size (i.e. at the scale of potentially smaller exposure areas) is assumed to be relatively low based on investigations of former golf courses and agricultural field areas where detailed data has been collected. Restriction of the default exposure area size to one acre also helps to ensure that unanticipated, small but heavily contaminated spill areas are captured by DU data (e.g., a former pesticide mixing area). Note that if a thorough Phase 1 ESA has not been completed, the default one-acre Exposure Area DU size may be judged inadequate for evaluation (this applies to all categories of large area sites discussed below).
Table 3-1 summarizes the recommended strategy for characterization of large parcels of land where localized spill areas are not known or anticipated. Division of the site into adjacent, one-acre DUs is recommended for areas 59 acres or less in size (Category 1, <59 Acres). Designation of DUs should reflect information garnered during the Phase I ESA to the extent practical (e.g., land-use history, terrain, soil type, etc.).
Random placement of 59, one-acre DUs is recommended for moderately large sites where the DUs will cover at least 50% of the total area (Category 2, <118 Acres). Testing of 59 of the total number of potential, one-acre DUs within the project area allows 95% confidence that the mean contaminant concentration in 95% of one-acre DUs at the entire site will be lower than the highest concentration reported in one-acre DUs that were tested (USEPA, 1989b). DUs should be placed in a systematic random distribution, and with consideration to adequately represent variability associated with land-use history, pesticide use, soil type, topography and other key factors gained from the Phase I ESA investigation. Note that the 95% confidence criteria will not be met if the highest mean concentration of just one of the 59 decision units exceeds the applicable target action level. Additional sampling would typically be required to adequately identify and address areas of the site with elevated contamination. Consultation with the HEER Office to discuss potential options is recommended.
Inclusion of baseline investigation data as described above is recommended for sites where 59, one-acre DUs will cover less than 50% but at least 10% of the total project area (Category 3, >118 to <590 Acres). The baseline investigation should be conducted first and will help to identify large-scale variance within the subject site and assist in subsequent DU placement and decision making. DUs should again be placed in a systematic random distribution, and with consideration to adequately represent variability associated with land-use history, pesticide use, soil type, topography and other key factors gained from the Phase I ESA investigation and the baseline investigation. For example, the baseline study might identify somewhat higher but still potentially acceptable levels of arsenic contamination in a portion of a field that was already under sugarcane production in the 1920s and 1930s. Placement of one-acre DUs within this area or even separate characterization of this area would be warranted. Including a baseline investigation also provides some level of data for the entire project area (in addition to the 59 one-acre DUs) and helps address concerns of prospective residents who understandably might ask about soil testing data for their area.
Confidence in the representativeness of data decreases as the total area encompassed by the one-acre DUs decreases. An increase in the number of one-acre DUs to 90 in addition to a baseline assessment is recommended for projects where less than 10% of the land will be covered by the DUs (Category 4; >590 Acres). This provides a 99% confidence that the mean contaminant concentration in 95% of one-acre DUs at the entire site will be lower than the highest concentration reported in the one-acre DUs that were tested (USEPA, 1989f).
Decision Units should be placed in a systematic random fashion, and with consideration to adequately represent variations in site characteristics (e.g., land-use history, terrain, soil type, etc.). The configuration of DUs across very large areas with respect to the planned redevelopment might also be desirable, although this could complicate usage of the data should redevelopment plans change in the future. HDOH feels that these recommendations are manageable in terms of the overall cost of large-scale, redevelopment projects. Alternative approaches should be discussed with HDOH on a case-by-case basis.
Return to the Top of the Page
C.2 Commercial and Industrial Sites
C.2.1 Small Spills
Figure C-1 depicts a small Source Area DU (150 ft2) for a suspected release of PCB oil at the edge of a former transformer pad. Staining on the pad suggested that it sloped to the side of where the DU was designated. An area extending approximately three feet out from the pad was designated for sample collection. Soil from 0 to 6 inches depth was targeted for sample collection. Triplicate 75-increment Multi Increment (MI) samples were collected. The flags denote the location of increments collected for the first sample (all flags not shown).
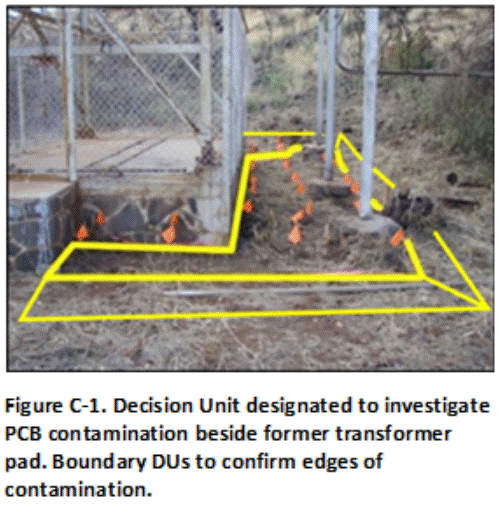
Three Boundary DUs were designated immediately adjacent to each the edge of the Source Area DU in anticipated clean soil in order to isolate contamination, if identified. Soil contaminated above a screening level appropriate for unrestricted reuse of the site will be excavated and disposed of in a regulated landfill. A similar confirmation sample would then be collected from the base of the excavation.
C.2.2 Commercial/Industrial Sites
Figure C-2 depicts DUs designated for a former electric power plant. A review of the site history, historical aerials and past discrete sample data suggested potential significant contamination of soil with PCBs in the area of the property where transformers were formerly stored and repaired. Three relatively small Source Area DUs were designated across this area (average 1000 ft2). A fourth larger Source Area DU was designated adjacent to these DUs in area where moderate contamination was possible. Small Boundary DUs were designated along the property boundaries of the suspect release area to potentially confirm an outer boundary of clean soil. The remainder of the property was divided into larger Exposure Area DUs appropriate for the current commercial use of the property. Significant PCB contamination was not anticipated in these areas.
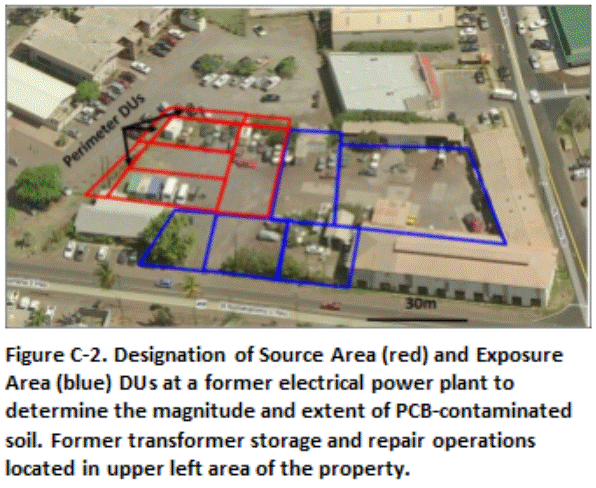
The investigation team concurred that the designated DUs would provide high data resolution for areas anticipated to require remediation while addressing potential direct exposure across the remainder of the site. Soil from 0 to 6 in depth was targeted for initial sample collection. Subsurface DU layers were to be designated in areas where PCB contamination was identified, with the resulting data to be used to design and optimize remedial actions.
C.2.3 Chemical Mixing and Storage Sites
Decision units designated for a former agricultural pesticide storage and mixing area are depicted in Figure C-3 and C-4. Relatively small (100 to 2000 ft2) Source Area DUs were designated in the former mixing tank area to evaluate potential leaching hazards posed by the triazine herbicides ametryn and atrazine (depicted in red, Figure C-3). The DUs were designated based on obvious or suspected areas of high contamination. For example, obvious or suspected release areas were identified on the ground under elevated mixing and storage tanks, under the floor or the storage building and in a low-lying drainage area adjacent to the tanks and building (Figure C-4). The use of small DUs helped assess potential leaching hazards from this area as well as optimized future remediation actions by minimizing the volume of potentially clean soil included in the DUs. The remainder of the mixing area, where low to moderate contamination was anticipated, was divided into larger Exposure Area DUs.
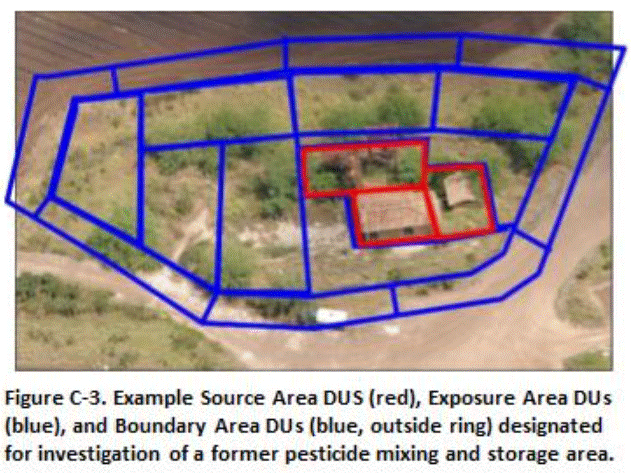
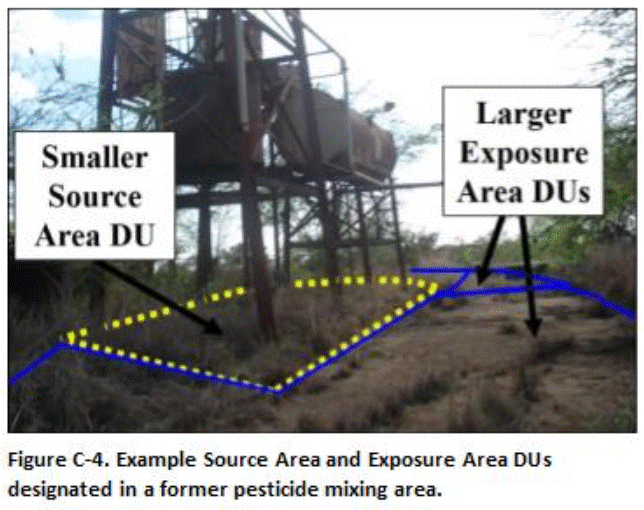 The upper 1 to 15 cm (0.4 to 6 in) of soil were initially tested in all DUs. The upper 0.5m (20 in) of soil was removed in Exposure Area DUs where contamination above screening levels was identified, with confirmation samples collected from the floors of the excavations. Heavy contamination identified under the tank area was later targeted for a more detailed subsurface DU-MIS investigation (see Section C.7).
The upper 1 to 15 cm (0.4 to 6 in) of soil were initially tested in all DUs. The upper 0.5m (20 in) of soil was removed in Exposure Area DUs where contamination above screening levels was identified, with confirmation samples collected from the floors of the excavations. Heavy contamination identified under the tank area was later targeted for a more detailed subsurface DU-MIS investigation (see Section C.7).
C.2.4 DU Designation Based on Redevelopment Plans
Figure C-5 depicts DUs for a proposed hotel development on a four acre site known to be contaminated with arsenic. The property was divided into four DUs, based on the proposed redevelopment layout and the suspected original location of a former arsenic mixing area. DUs A through C represent exposure areas in anticipated cleaner areas of the property. A smaller Source Area DU (DU-D) was designated in the suspect mixing area in order to help isolate soil anticipated to be most heavily contaminated and optimize remediation cost.
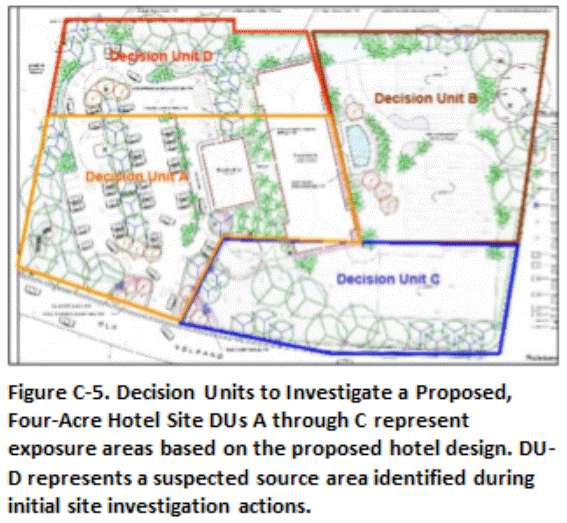 Soil in DUs that failed the cleanup level for arsenic was to be excavated and disposed of at a regulated landfill. A series of subsurface DU layers was designated in DU-D to a depth of ten feet to identify the depth of contamination and to serve as confirmation sample data for anticipated soil removal. The upper 0.5 yd of soil was to be removed in DUs A-C if the cleanup level was exceeded, with follow-up confirmation samples to be collected from the floors of the DUs. Confirmation samples were to be screened in the field with a portable XRF prior to submittal to a laboratory to expedite soil removal and completion of the project.
Soil in DUs that failed the cleanup level for arsenic was to be excavated and disposed of at a regulated landfill. A series of subsurface DU layers was designated in DU-D to a depth of ten feet to identify the depth of contamination and to serve as confirmation sample data for anticipated soil removal. The upper 0.5 yd of soil was to be removed in DUs A-C if the cleanup level was exceeded, with follow-up confirmation samples to be collected from the floors of the DUs. Confirmation samples were to be screened in the field with a portable XRF prior to submittal to a laboratory to expedite soil removal and completion of the project.
Return to the Top of the Page
C.3 SINGLE-FAMILY HOMES
Soil contamination concerns associated with residential properties often focus on the presence of lead-based paint residue around the immediate perimeter of homes constructed prior to the mid-1970s. Soil under and around homes constructed prior to this time might also have been treated with organochlorine termiticides (e.g., Technical Chlordane) or even arsenic.
Figure C-6 depicts typical DU designation to investigate these potential concerns. A narrow Source Area DU (or DUs) was designated around the immediate perimeter of the home, typically within 1.0 m (1.09 yd) to 1.5 m (1.64 yd) out of the foundation. This could also be classified as an Exposure Area DU, since landscaping and other attractions around the perimeter of a house can attract young children. There is high confidence that the DU will capture any contamination present based on experience at other homes, and additional, Boundary DUs to help confirm the boundary with clean soil are assumed to not be necessary. The remainder of the yard was designated as a single large Exposure Area DU. No contamination in this area is anticipated. The upper 0 to 15 cm (0.4 to 6 in) of soil was targeted for sample collection.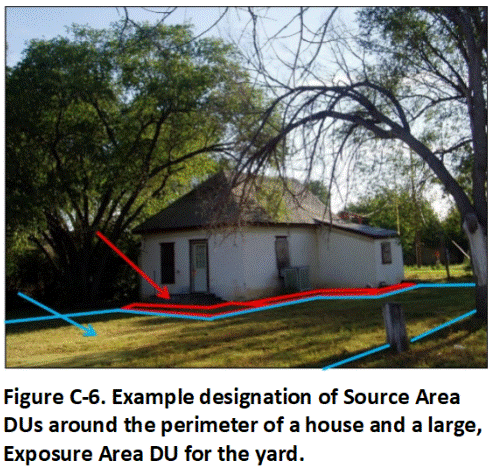
Soil that exceeds target, residential screening levels is to be excavated and removed, and a confirmation sample collected from the floor of the excavation. Each side of the house could be designated as a separate DU for testing if there is a reason to think that these areas could be different. Landscaping or a small garden might be present one side of the house. The side of the house that faces the sun most of the day might be more susceptible to weathering of lead-based paint. Multiple DUs could similarly be designated for the yard if areas of the yard are used for different purposes and could be classified as separate exposure areas (e.g., play areas or garden areas).
A proper MI sample could not be collected under the slab of the house due to the absence of a crawl space and the effort required to penetrate the slab and avoid utilities. As an alternative, soil cores representing DU layers were collected and tested from three Exploratory Boreholes drilled through the slab (0 to 25 cm, 25 to 50 cm, 50 to 100 cm (1 to 10, 10 to 20, 20 to 39 in); refer to Section 3.6.4). Data from the cores was to be used to establish the presence, but not necessarily the absence of treated soil under the slab. The slab area was later designated as a separate DU and tested following demolition and removal of the structure (refer to Example C.7.3).
Separate testing of each structure might not be practicable or necessary for characterization of large neighborhoods where dozens or even hundreds of houses are scheduled for demolition (e.g., large military bases). In such cases, the neighborhood can be divided into clusters of homes constructed during the same time period and by the same builder, or otherwise with the assumption that the use of lead-based paint or termiticides around the buildings would be similar. Detailed characterization could be carried out for a select number of buildings within each cluster (e.g., 10-20%). The results can then be applied to the remainder of the buildings to prepare initial soil management plans. More detailed testing can be carried out as needed to confirm conclusions drawn from the initial data. This includes testing of stockpiled soil prior to reuse or disposal.
Return to the Top of the Page
C.4 HIGH-DENSITY HOUSING
The investigation of large, high-density residential areas for potential soil contamination concerns is approached in a similar manner as done for individual homes. Suspect source areas of significant contamination are targeted as separate Source Area DUs for characterization. The remainder of the property is divided into larger, Exposure Area DUs.
Figure C-7 depicts DUs designated for a public housing complex suspected of being constructed in an area where pesticides were mixed and stored in the past. Soil immediately adjacent to a retaining wall at the bottom of the edge of the complex was anticipated to be at greatest risk of contamination. Small 500 to 1000 ft2 Source Area DUs were designated along the retaining wall based on the location of apartment patios and use of the area for landscaping or small gardens (DUs 1, 2, 3, 8, and 12). Larger, Exposure Area DUs based on open, grassed areas or play areas were designated around nearby buildings.
The upper 0 to 15 cm (6 inches) interval of soil was targeted for initial sample collection. Due to the ongoing use of the complex for housing, DU areas with concentrations of pesticides that exceed residential screening levels were to either be capped with clean soil or, where feasible, the upper one foot of soil removed and replaced with clean soil. Testing of soil under buildings was to be carried out in the future when redevelopment of the property takes place.
Return to the Top of the Page
C.5 SCHOOLS
Designation of DUs for characterization of potential soil contamination at schools typically represents a combination of approaches used for commercial/industrial facilities and high-density residential complexes. Designation of Source Area and Exposure Area DUs for sample collection might include potential lead-contaminated or termiticide-treated soil around perimeters of older buildings, garden areas where persistent pesticides might have been used in the past, barren areas of soil in areas frequented by children and staff and areas where soil is discovered to contain pieces of wire, porcelain, melted glass and ash indicative of past burning.
Figure C-8 depicts DUs designated for a school to test for the presence of lead-contaminated soil associated with burning and dumping prior to construction of the campus. The lower campus area is especially considered at risk of contamination. A soccer/football field, school garden and an area under trees where students congregate are designated as separate Exposure Area DUs. Soil in a second area under trees where students congregate is discovered to contain bits of porcelain and melted glass and is designated as a combined Source Area-Exposure Area DU.
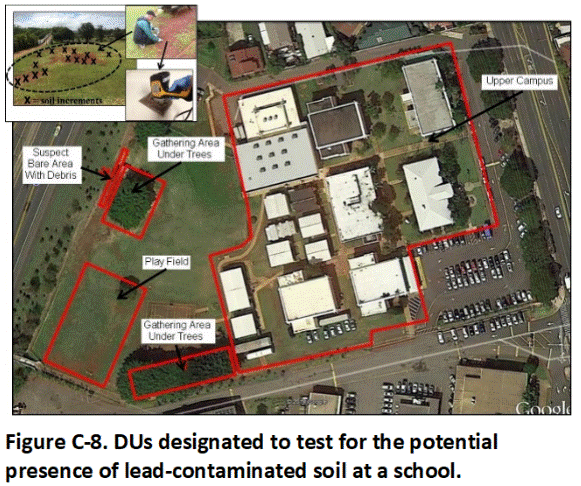
The upper campus is considered low risk for contamination and treated as a single DU area. Focus is paid to barren areas of soil exposed in otherwise thick lawns, including soil along walkways, under outdoor tables and in areas of high foot traffic.
Sample increments were collected from the upper 1 to 15 cm (6 in) of soil in each DU. Field screening of combined increments from clusters of barren areas within the main campus was carried out using a portable XRF to determine if large-scale patterns of contamination could be distinguished. The soil was ultimately combined and tested as a single sample after field screening suggested low levels of lead within the DU area as a whole.
Return to the Top of the Page
C.6 LARGE SINGLE-USE AREAS
Characterization of very large areas that have historically been used for a single purpose is sometime necessary as part of a redevelopment project. Examples include large, former agricultural fields, fishponds, former military munitions testing and training areas or golf courses to be redeveloped for residential housing or commercial use. Testing of soil might be carried out to investigate risk associated with existing contamination or to establish a baseline for future redevelopment and protection of the area.
Two relatively simple examples are given below, redevelopment of a former golf course and former agricultural field for residential housing. An example investigation of a more complex, former industrial complex is presented in Section C.9.
C.6.1 Former Golf Course
In this example, a 250 acre former golf course is slated for redevelopment as residential housing (Figure C-9). Arsenic and other pesticides were known to have been used for weed control in the past. Several hundred homes were to be constructed on the property. Experience at other golf courses suggested that the upper 1.5 ft of soil could be impacted above levels of potential concern.
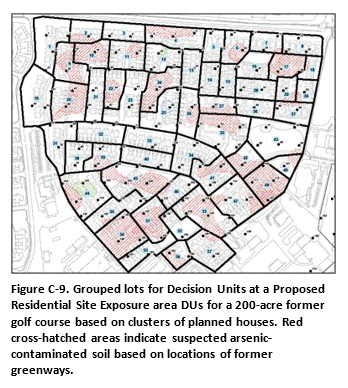
Testing of each individual lot was determined to be impractical and, given the relatively uniform use of pesticides across the course over time, unnecessary. As an alternative, clusters of four to five homes were designated as DUs for testing (total 57). Three layers were designated for testing at each DU: 0 to 15 cm, 15 to 50 cm and 50 to 100 cm (0-6, 6-20, 20-39 inches). A thirty-increment sample was to be collected from each DU layer.
A backhoe was used to dig 30 three-foot-deep pits in each DU in a systematic, random fashion (Figure C-10). A single increment was collected for each layer by scraping 50 grams of soil from the entire, exposed interval of soil. Layer-specific increments were combined and three separate samples prepared for each DU. Independent triplicate samples collected from separate pits were collected and prepared for six of the 59 DUs and used to test the precision of the overall sampling method. The resulting data allowed a three-dimensional image of soil that exceeded cleanup levels to be developed and incorporated into the site grading and soil removal plan.
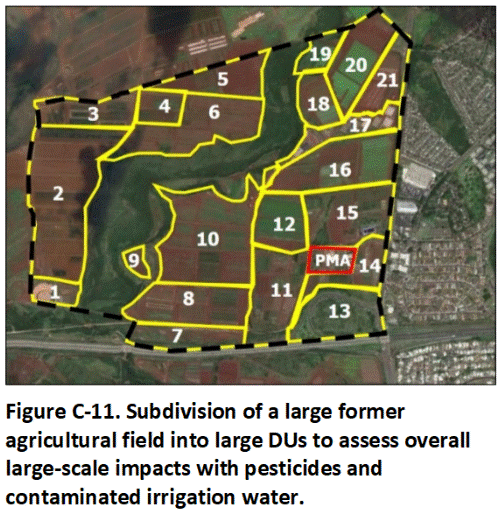
C.6.2 Former Agricultural Field
This example focuses on the redevelopment of a 500-acre former agricultural field proposed to be redeveloped for residential housing (Figure C-11). The proposal calls for 1,000 single-family homes to be constructed on 10,764 ft2 lots, with additional space allotted for small parks and playgrounds. The exact layout of the development plans has not been finalized, however.
 The developer decides to investigate the fields prior to purchasing the property. The fields are investigated in three phases. The first stage of the investigation includes a thorough review of the history of the property, including soil types, drainage patterns, drainage patterns, types of crops grown and pesticides used, potential presence of localized pesticide mixing and storage areas, etc. The second stage involves completion of a large-scale “baseline” characterization of pesticide levels between individual field areas. The third stage involves random testing of a statistically significant number of hypothetical house lots randomly located throughout the field area.
The developer decides to investigate the fields prior to purchasing the property. The fields are investigated in three phases. The first stage of the investigation includes a thorough review of the history of the property, including soil types, drainage patterns, drainage patterns, types of crops grown and pesticides used, potential presence of localized pesticide mixing and storage areas, etc. The second stage involves completion of a large-scale “baseline” characterization of pesticide levels between individual field areas. The third stage involves random testing of a statistically significant number of hypothetical house lots randomly located throughout the field area.
The results of the baseline assessment can be used by the developer to decide whether to proceed to a more time consuming and costly, detailed investigation. The baseline investigation also required a thorough walkthrough of the entire site. This can assist in identification of previously unknown dumping sites, waste pits, former plantation camp areas, pesticide mixing or storage areas, etc. that might otherwise be missed. A baseline assessment combined with a thorough Phase 1 Environmental Site Assessment (Phase 1 ESA) report might also be adequate for regulatory concurrence to develop a field area for commercial/industrial use without the need for higher-resolution data.
Phase 1: Background Investigation
The Phase 1 ESA included a review of the following information:
- Crop history;
- Current and past pesticide use;
- Historic aerial photographs;
- Historic Sanborn Fire Insurance maps, topographical maps, or other maps (used to identify past buildings and pesticide mixing and storage sites);
- Interviews with former employees;
- Existing soil investigation reports (including investigations of adjacent or nearby fields using lot-size DUs);
- Review of other published, historic information (journals, etc.); and
- Field inspection (current operations, former buildings, suspect dump areas, etc.).
The Phase 1 ESA review determined that the fields were only used to grow crops. A former, pesticide mixing area in lower right of area (red) to be investigated separately from fields due to suspected areas of localized, heavy contamination (see Figure C-11; refer also to Example C.7.2). There was no additional evidence of past, on-site mixing or storage of pesticides or reasons to otherwise suspect the presence of small, localized areas of heavy contamination.
Phase 2: Baseline Investigation
Decision Units for the baseline investigation were designated based on soil type, crop history, pesticide use, potential use of contaminated groundwater or surface water for irrigation, terrane and drainage patterns, etc. A total of 21 DUs were designated (see Figure C-11). A 50-increment, 2 to 3 kg, sample was collected from each DU. The upper 0 to 15 cm (6 in) of soil was targeted for sample collection. The exposed soil is assumed to be reasonably representative of the upper 50 cm (20 in) due to regular plowing of the fields, as determined in the Phase 1 assessment. Independent, triplicate samples (primary sample plus two replicates) were collected in two of the DUs to test the precision of the overall sampling method. A total of 25 samples were collected, including the replicates. The fields had been recently plowed, allowing for easy sample collection. Field work was completed over the course of one week.
The results of the baseline investigation suggested relatively low levels of residual pesticides throughout the fields. A decision was therefore made by the potential developer to proceed to more detailed testing at the scale of hypothetical, individual house lots.
Phase 3: Testing of Housing Exposure Area DUs
The baseline assessment can be thought of as testing of the fields at a “neighborhood” scale. Each of the 1,000 house lots planned for the redevelopment could be considered to represent an Exposure Area DU within a neighborhood. Testing of every lot is not practicable. After consulting with risk assessors trained in Gy’s Theory of Sampling, a decision is made to test a sufficient number of lots to conclude with 95% confidence that the concentration of a pesticide does not exceed the target screening level for at least 95% of the lots in total.
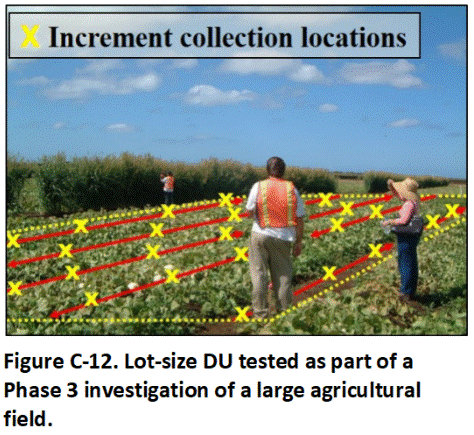 This was accomplished by testing of 59, randomly located, hypothetical, 10763 ft2 Exposure DUs within the project area (Figure C-12; , USEPA 1989b; see also, HIDOH 2016c). An attempt was made to space the DUs in systematic random distribution within the field area as a whole. This allowed 95% confidence that the concentration of a contaminant in at least 95% (950) of the proposed 1,000 house lots will be lower than the highest concentration reported for the 59 lots tested. If the highest concentration reported for any given pesticide does not exceed the correlative screening level, then the field can be cleared for residential redevelopment. This was the case for the Phase 3 study and a decision was made to proceed with redevelopment.
This was accomplished by testing of 59, randomly located, hypothetical, 10763 ft2 Exposure DUs within the project area (Figure C-12; , USEPA 1989b; see also, HIDOH 2016c). An attempt was made to space the DUs in systematic random distribution within the field area as a whole. This allowed 95% confidence that the concentration of a contaminant in at least 95% (950) of the proposed 1,000 house lots will be lower than the highest concentration reported for the 59 lots tested. If the highest concentration reported for any given pesticide does not exceed the correlative screening level, then the field can be cleared for residential redevelopment. This was the case for the Phase 3 study and a decision was made to proceed with redevelopment.
Note that the 95% confidence criteria will not be met if the highest mean concentration of just one of the 59 DUs exceeds the applicable target action level (see HIDOH 2016c). Additional sampling would instead be required to adequately identify and address areas of the field where lot-size DUs could fail screening levels. In most cases this would not be cost-effective and redevelopment for less sensitive purposes would need to be considered.
Return to the Top of the Page
C.7 VERY SMALL AREAS
Characterization of DUs as small as a 10 ft2 or less in area and a very small volume of soil or sediment might be required under some circumstances. Examples include testing of the upper few inches of soil around a leaking tank valve to document the presence of a release (Figure C-13) or testing of sediment in a storm sewer vault to assess runoff from a known or suspect, contaminated area (Figure C-14). The third example reflects collection of a soil sample from an obviously contaminated location within a large, illegal dump site for enforcement purposes to document the presence and nature of the chemicals released (Figure C-15).
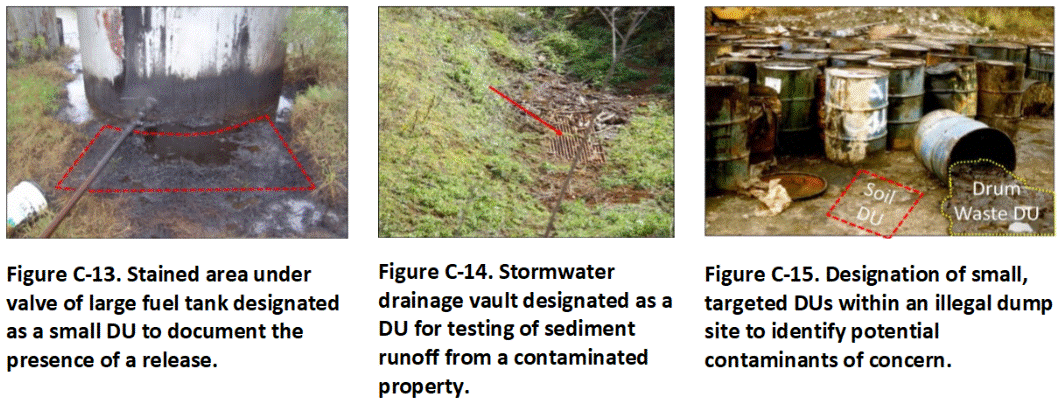 Figure C-13. Stained area under valve of large fuel tank designated as a small DU to document the presence of a release. Figure C-14. Stormwater drainage vault designated as a DU for testing of sediment runoff from a contaminated property. Figure C-15. Designation of small, targeted DUs within an illegal dump site to identify potential contaminants of concern.
Figure C-13. Stained area under valve of large fuel tank designated as a small DU to document the presence of a release. Figure C-14. Stormwater drainage vault designated as a DU for testing of sediment runoff from a contaminated property. Figure C-15. Designation of small, targeted DUs within an illegal dump site to identify potential contaminants of concern.
These examples are similar to the concept of “judgmental,” “biased” or “subjective” sampling but with the term “discrete” referring to a small, localized area targeted for characterization rather than the method used to collect a sample. The distinction between testing of “discrete areas” versus collection of “discrete samples” from a single point within a targeted area is important and a common cause of confusion past environmental investigation guidance documents (refer to Appendix E).
In the case of the storm sewer vault, the volume of sediment in the vault represents the DU of interest. A minimum 30-increment, 1 to 3 kg sample is collected. If the volume of sediment is small enough, then the entire DU can be collected and submitted to the laboratory for processing and testing. This ideal scenario, referred to as to as “direct inference” (AAFCO 2015), negates the need for the collection of a MI samples (and replicates) and eliminating potential field error (e.g., <2 kg of material present). The sediment must be processed and subsampled for analysis at the laboratory in accordance with MI protocols for the data to be considered representative.
The concept of very small DUs also applies to targeted “DU Layer” of soil or sediment in a core where decisions are to be made on data for single, exploratory boreholes. If the targeted interval of the core is less than three feet in length, then it is usually practical and even desirable to submit the entire core interval to the laboratory for processing and testing. In other cases, subsampling of the core will be required to reduce the sample to a manageable mass (see Appendix G).
Return to the Top of the Page
C.8 SUBSURFACE DECISION UNITS
C.8.1 Buried Waste Pit
This example demonstrates the designation of subsurface DU layers for testing at a former industrial site slated for residential and commercial redevelopment. Four distinct layers were identified in the sidewall of an excavation to remove what was initially thought to be a small dump site (Figure C-16). DU Layer 4 represents native soil. DU Layer 3 represents a former waste pit. DU Layer 2 is fill material placed over the former dump that was contaminated by subsequent releases at the surface. DU Layer 1 represents a thin layer of more recent and presumably clean fill.
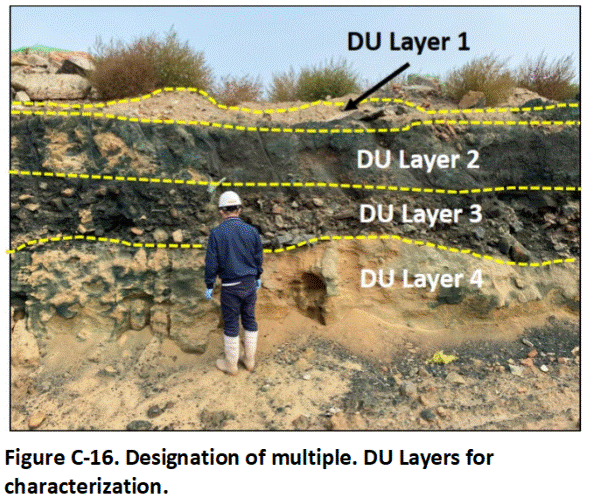 A single, 2 to 3 kg, 75-increment sample was collected from each DU layer to obtain preliminary data on the type and general magnitude of contamination present. This comes with an understanding that contamination can vary significantly between different areas of the waste pit.
A single, 2 to 3 kg, 75-increment sample was collected from each DU layer to obtain preliminary data on the type and general magnitude of contamination present. This comes with an understanding that contamination can vary significantly between different areas of the waste pit.
The resulting data suggested that the underlying, native soil in DU Layer 4 and the fill material in DU Layer 1 were relatively clean. Significant contamination of DU Layer 2 and DU Layer 3 with a mix of heavy metals and solvents was confirmed, however.
A decision was made to remove DU Layers 2 and 3 prior to redevelopment of the property. Exploratory trenches and borings were used to identify the approximate lateral and vertical extent of the waste pit material. Adequate in situ samples were collected to permit preparation of a workplan to excavate the soil and transport it to a treatment and disposal facility. The thickness of targeted DU layers varied across the site. Refer to Appendix G for guidance on the collection of representative samples under this scenario. Confirmation samples were collected from the floor and sidewalls of the excavation to confirm cleanup to screening levels for residential redevelopment (see Appendix H).
C.8.2 Above Ground Storage Tanks
This example expands on characterization of the former, pesticide mixing area discussed in Example C.1.3 (see Figure C-4). Contamination of soil in the outer areas was anticipated to be restricted to the upper three feet of soil. Arsenic and dioxin were the primary contaminants of concern, posing potential direct exposure risks. The area was subdivided into multiple, Exposure Area DUs an areas of no more than 5000 ft2, the default exposure area used to clear site for residential redevelopment.
Soil beneath tanks used to store and dispense pesticides was suspected to be heavily contaminated at depth with arsenic, dioxins and triazine pesticides, posing both direct exposure and leaching hazards. Source Area DUs designated in this area were intentionally made no larger than 1000 to 2000 ft2 to provide a higher resolution of sample data and optimize anticipated remedial actions.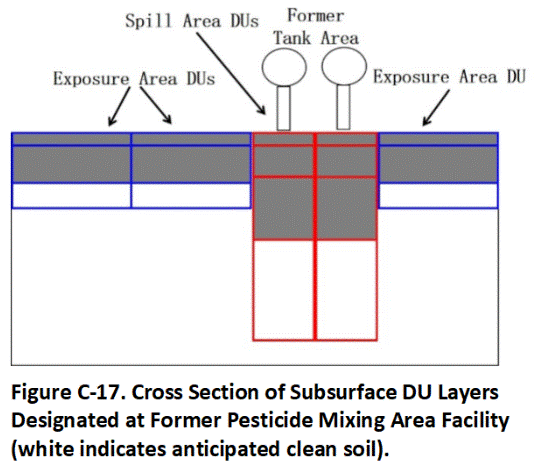
Subsurface DU layers designated for the DU areas are depicted in Figure C-17. Three vertical DU layers were designated for the outer Exposure Area DUs: 0 to 12″, 2) 12″ to 36″ and 3) 36 to 54″. The in situ volume of soil associated with the respective layers in each of the 5000 ft2 DUs was approximately 185 cyds, 370 cyds and 275 cyds, respectively. Removal of the upper two layers across most of the area was anticipated. The lowermost layer was anticipated to be clean.
Four vertical DU layers were designated to characterize soil in Source Area DUs designated beneath the former aboveground storage tanks (see Figure C-16): 1) 0-1′, 2) 1-3′, 3) 3-5′ and 4) 5-10′. The volume of soil associated with the respective layers ranged from 25 to 150 cyds for the first two layers and 150 to 500 cyds for the lower layers. The deepest layer in each DU was anticipated to be relatively clean.
A 30-increment sample was collected from each DU layer using a direct-push rig (Figure C-18). Each interval of the targeted DU layer in the collected core represented a sample increment. Individual increments were too large to combine into a single sample for a DU layer as a whole. As an alternative, a 50-gram subsample was collected from each increment. Increments for a corresponding DU layer were combined to prepare a single, 1.5 kg MI sample for the layer.
Field DU replicate samples (triplicates) were collected from independent borings for each DU layer in one of the Exposure Area DUs. A single set of field subsample replicates was collected from cores associated with one of the samples. The small size of the Source Area DUs under the tanks precluded the collection of field DU replicate samples in this area.
Return to the Top of the Page
C.9 STOCKPILES
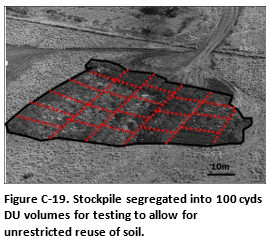 Refer to Appendix H for detailed guidance on testing soil stockpiles. Figure C-19 depicts a 2,500 cyds stockpile proposed for reuse as fill material in a residential development project. After discussing with risk assessors, the stockpile was flattened to no more than a three feet thick and divided into 25 100 cyds DU volumes for individual testing. This equates to the volume of soil placed in a hypothetical 5000 ft2 exposure area to a depth of 1 ft, for example a small playground or open lawn area.
Refer to Appendix H for detailed guidance on testing soil stockpiles. Figure C-19 depicts a 2,500 cyds stockpile proposed for reuse as fill material in a residential development project. After discussing with risk assessors, the stockpile was flattened to no more than a three feet thick and divided into 25 100 cyds DU volumes for individual testing. This equates to the volume of soil placed in a hypothetical 5000 ft2 exposure area to a depth of 1 ft, for example a small playground or open lawn area.
A single, 1 to 3 kg, 50-increment sample was collected from each DU. Replicate (triplicate) samples were collected in three of the DUs. Soil that met screening levels for unrestricted land use was cleared for use as fill material in the development. Soil that did not meet screening levels for unrestricted land use was disposed of at a regulated landfill.
Return to the Top of the Page
C.10 BUILDING DEMOLITION
C.10.1 Example Setting
In this example, a former manufacturing building is to be demolished and the property remediated for commercial redevelopment (Figure C-20). All windows, doors, electrical equipment and wiring, lighting fixtures and similar material are to be removed from the building, leaving only the concrete structure itself. Asbestos containing tiles and piping insulation are to be removed and disposed of separately. Soil under the building must also be tested for organochlorine pesticides to determine if it can be reused as fill material on site or must be disposed of in a landfill.
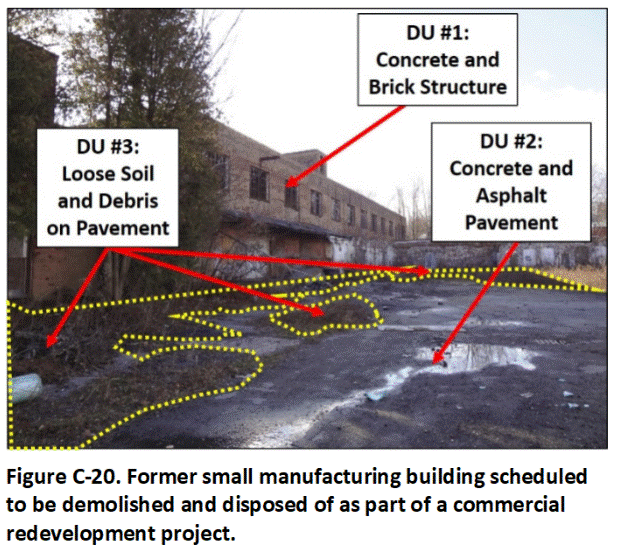 The concrete interior and exterior walls, floors and ceilings of the building are known to have been sealed with PCB- and lead-based paint. Paint was observed to have flaked off in several locations both inside and outside of the building. Dirt and debris on concrete and asphalt pavement around the building are suspected to be contaminated with chips of PCB- and lead-based paint.
The concrete interior and exterior walls, floors and ceilings of the building are known to have been sealed with PCB- and lead-based paint. Paint was observed to have flaked off in several locations both inside and outside of the building. Dirt and debris on concrete and asphalt pavement around the building are suspected to be contaminated with chips of PCB- and lead-based paint.
Recycling of the building concrete is not allowed unless the paint and caulking are first removed. Caulking around windows is suspected to be PCB-based and will be removed to the extent practicable and tested separately for disposal prior to demolition of the building. Any remaining caulking is assumed to be di minimis in terms of the total bulk mass of the building and will not require addition action or testing. Removal of the paint to allow recycling of the building was determined to not be cost effective, however.
Testing and a Hazardous Waste Determination was required for disposal of the demolition debris in a regulated, municipal landfill. Four DUs were designated for sample collection (see Figure C-20): 1) The concrete structure, 2) The surrounding concrete and asphalt, including an adjacent parking lot, 3) Loose dirt and other debris on the pavement and 4) Soil immediately under the slab of the building. Each requires a separate sampling strategy.The building is to be tested as a single unit, including the concrete and any paint adhered to the concrete. Note that testing of bulk building debris prior to disposal in a regulated, municipal landfill might or might not be required in a specific area. Check with the local regulatory agency for details. The examples below apply to hypothetical circumstances where the collection and testing of samples of building debris is in fact required.
C.10.2 Testing of Bulk Building Debris
The Investigation Question tied to testing of the building is: “What is the concentration of PCBs and lead for the collective walls, floors and ceilings of the building as a whole?” Note that the concentration of PCBs and lead in the paint itself is not the focus of the investigation and does not need to be considered, other than noting the potential presence of these compounds.
Two options to answer this question were discussed. The first option involved estimating the total mass of PCBs and lead in the paint and dividing this by the estimated mass of the building itself. This could be accomplished through the collection of a 75-increment sample of the paint in a systematic, random fashion throughout the interior and exterior of the building. The total mass of PCBs and lead present in the paint is calculated by dividing the sample data by the estimated, total mass of paint on the building. The mass the building is estimated based on the total area and thickness of the floors, walls and ceilings and the average density of the concrete. Two additional, replicate samples of the paint would be collected to test the overall reproducibility of the sampling method.
The second option involved the collection of 75 core increments in a systematic random fashion through the entire thickness of the floors, walls and ceilings. This approach allows direct collection of a representative sample of the bulk material prior to demolition. Care would be taken to ensure that paint on either side of the structure is included in the increment collected. Increments would be combined to prepare a single sample for the building as a whole. Two additional, replicate sets of core increments would be independently collected from different areas of the structure to test the overall reproducibility of the sampling method.
Based on local regulations, the building can be disposed of in a municipal landfill provided that the concentration of PCBs for the structure as a whole is less than 50 mg/kg and the concentration of lead is less than 100 mg/kg. if the concentration of lead exceeds 100 mg/kg, then an additional leaching test must be carried out on the sample(s) as part of the Hazardous Waste Determination. This is referred to as the Toxicity Characteristic Leaching Procedure or “TCLP” test. If the TCLP data exceed 5 mg/L total lead, then the building debris must be disposed of in a hazardous waste landfill.
The developer for the project ultimately chose to collect MI core increment samples for characterization of the concrete structure. Resulting data confirmed that the demolition debris could be disposed of in a local, municipal landfill.
C.10.3 Testing of Concrete and Asphalt Pavement
Field testing with a portable XRF verified that yellow striping on the asphalt and concrete pavement was lead-based. The lead is tightly bound within the thermoplastic, polymer paint and is not significantly leachable unless ground. Testing of the pavement for disposal will therefore not be required provided that the debris is disposed of in a regulated, municipal landfill. As an alternative, the asphalt can be included in the feedstock at an asphalt production plant without further testing.
Recycling of the concrete and asphalt pavement for general, unrestricted reuse as fill material would not be allowed unless the striping is first removed. This would require milling of the striping from the pavement and likely disposal of the material as hazardous waste due to failure of TCLP limits for lead. This was determined to be economically practical for the smaller area of concrete pavement but not for the larger asphalt pavement area.
Asphalt pavement was ultimately removed and sent to a bulk asphalt plant for recycling. Concrete pavement was sent to a recycling facility after removal of striping and ground for reuse as gravel base fill.
C.10.4 Testing of Sweep Material
Dirt and other debris is to be swept from the pavement and placed in a single stockpile on site and tested for disposal. The total volume of material stockpiled is determined to be less than 100 cyds and sufficiently small for testing as a single DU (refer to Section 3.3.4). A single, 75-increment sample is collected and tested for PCBs, lead, PAHs and heavy oil. Two additional, replicate samples are collected and tested to assess the precision of the overall sampling method.
The material can be disposed of in a municipal landfill provided that the concentration of PCBs is less than 50 mg/kg and provided that lead in leachate from the sample does not fail the Toxic Characteristic Leaching Procedure (TCLP) limit of 5 mg/L. The debris must otherwise be disposed of in a hazardous waste landfill.
C.10.5 Testing of Subslab Soil
Five Exploratory Boreholes (see Appendix G, Section G-1) were installed through the slab of the floor prior to demolition of the building. Discrete core samples were independently tested to screen for the presence of organochlorine termiticides in the underlying soil. High levels of Technical Chlordane were identified in some but not all samples. This is not unexpected, since termiticides are normally only applied to soil within and around utility trenches that can serve as conduits for termite invasion. Identification of the exact locations of utility trenches to help isolate treated soil after a building has been demolished is difficult and the entire subslab area is normally tested as a single or multiple DUs.
Termiticide-treated soil is typically confined to a depth of 12 to 24 inches below the base of a slab. Three subsurface layers were therefore targeted for testing: Layer A (0 to 12″), Layer B (12 to 24″) and Layer C (24 to 48″). The objective was to isolate heavily contaminated soil in the upper one to two layers of soil with the third, deepest layer anticipated to meet screening levels.
A maximum DU volume of 500 cyds was required by the overseeing regulatory agency for onsite reuse of the soil as part of the commercial redevelopment (refer to Section 3.3.4). A maximum DU volume of 100 cyds was required for unrestricted, offsite reuse of the soil.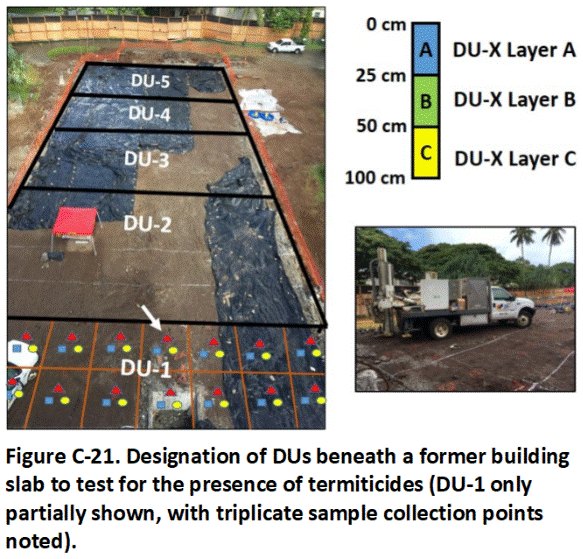
The former slab area was subsequently divided into five DU areas of 4000 ft2 each (Figure C-21). Three layers within each DU designated for individual testing in accordance with the above-noted scheme (e.g., DU-1A, DU-1B, DU-1C; etc.). This allowed the maximum acceptable DU volume for unrestricted, offsite reuse to be met for Layers A and B if applicable screening levels were met. Soil from Layer C could be reused onsite provided that screening levels for commercial land used were met.
Termiticides are normally dissolved in a solvent and poured or sprayed onto soil in order to obtain a relatively uniform application. Based on anticipated low distributional heterogeneity, 30-increment MI samples were therefore deemed appropriate.
A push rig was used to collect thirty, 100 cm (39 in) cores in a systematic, random fashion from each DU. Targeted DU layers were identified in each core and a 50-gram subsample collected. Subsamples from each core were combined to prepare a 1.5 kg MI sample for a DU layer (refer to Appendix G, Section G.3). Triplicate samples were collected in DU-1 in order to test the precision of the overall sampling method, for a total of 17 samples (refer to Figure C-21).
In this example, the uppermost two layers of soil in all of the DUs was determined to be too heavily contaminated with Technical Chlordane for either onsite or offsite reuse. Data for TCLP tests carried out on the three most contaminated samples indicated that the soil was not classifiable as hazardous waste and the soil was subsequently disposed of at a municipal landfill. Data for Layer C in all DUs met screening levels for onsite reused of the soil as fill material, if needed.
Return to the Top of the Page
C.11 LARGE INDUSTRIAL COMPLEXES
C.11.1 Overview
Characterizing large former industrial complexes for cleanup and redevelopment can be challenging. Projects can be several square miles in size and include multiple types of manufacturing facilities, buried waste pits, worker housing and office buildings. The scope of potential environmental concerns will reflect the types of chemicals made and used at the site and typically includes direct exposure to contaminants in soil (e.g., heavy metals, PCBs, dioxins, organochlorine pesticides), leaching and contamination of groundwater or nearby surface water (e.g., soluble pesticides and solvents) and vapor emissions to outdoor and indoor air (e.g., solvents and fuels). Gross contamination issues, including short-term but strong vapor emissions from heavily contaminated soil as well as sheens in runoff and fouling of equipment (e.g., petroleum). Contamination between release sites might come mixed, making the source difficult to identify.
Preparation of a thorough Phase I is critical for development of an efficient and reliable site characterization plan (refer to discussion in Example C.5.2). This should include a thorough review of the site history, including interviews with current and former workers, available manufacturing and property records, fire insurance maps, historical aerial photos, site walkthroughs and data and observations from preliminary borings and test pits. The resulting information can be used to help designate DUs for sample collection. The review can in many cases also help expedite clearance of large portions of the site for redevelopment, while additional resources are gathered to address localized contamination problems.
A useful first step in initiating discussions for the investigation of a very complex, large area is to propose designation of the entire site as a single DU for the collection of a single sample. This is unlikely to be satisfactory for either risk assessors or remediation experts. As described in the example below, the site is then progressively subdivided into smaller Exposure Area and Source Area DUs until the resulting data will be acceptable to all parties for final decision making. This approach requires more upfront time before samples can be collected in the field, but will significantly expedite completion of the project.
C.11.2 Former Textile and Metal Alloy Manufacturing Complex
Background
This example presents a hypothetical 40-acre former textile and metal alloy complex area that will be demolished and redeveloped for residential apartments and office buildings (Figure C-22). The site consists of a former factory area, a former worker housing area and a former office building area. All buildings have been demolished and removed including the foundations and slabs.
Site records were reviewed and interviews carried out with past workers who had knowledge of the use of hazardous chemicals at the factories. Contamination of both surface soil and subsurface soils with heavy metals and solvents was suspected throughout the former factory areas. Contamination in the former worker housing area and office building area was assumed to be limited to the past use of lead-based paint and use of organochlorine pesticides for termite control under and around buildings.
Investigation Approach
The sampling team worked with risk assessors and remediation experts to develop a series of site investigation questions regarding environmental risk and optimization of anticipated remediation. Division of the site into DUs for sample collection and characterization would continue until the data to be collected met the needs of all stakeholders. Exploratory borings, trenches and pits would be used for initial investigation of suspect contaminated areas to assist in designation of DUs.
Fifty-increment samples were to be collected to test surface soil DUs as a default. Thirty-increment samples could be collected to test soil contaminated by solvents, petroleum and other releases of liquid wastes. Seventy-five increments were to be collected in cases where the contaminant could be present in the form of small ships and nuggets. Thirty-increment samples were to be collected to test subsurface DU layers, with the understanding of a reduced reliability in the resulting data for soil contaminated with particulate matter.
Field replicate samples were to include DU triplicates (primary plus two independent replicates) collected from 10% of all surface soil DUs. Field subsample triplicates were to be collected from 10% of tested subsurface DU layers. Laboratory subsample triplicates were to be tested for 10% of the total samples submitted for testing. Minimum 50-increment confirmation samples would be required for all excavations.
Decision Unit Designation
Four options were discussed by risk assessors and remediation experts for division of the site into DUs for testing (Figure C-23). Designation of DUs was to be continued until the resulting data would adequately address all site investigation questions and concerns.
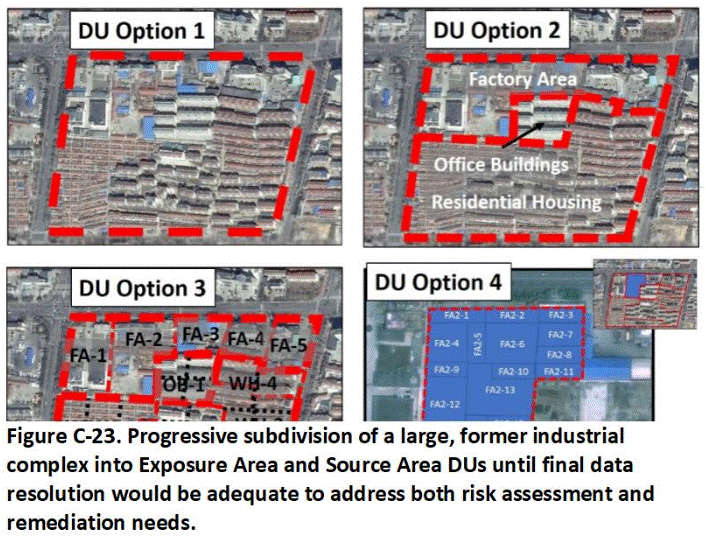
Option 1:
To initiate discussion, a proposal was made to designate the entire, 40-acre site as a single Exposure Area DU (Option 1 in Figure C-23). If this option was accepted, then a single surface soil sample would be collected, with two additional, replicate samples collected to test overall data precisions. The risk assessors concluded that the area was too large to treat as a single, exposure area. The remediation experts also stated that the data would not be adequate to address known areas of heavy contamination in the former factory area.
Option 2:
As a second alternative, the former worker housing, office building and factory areas are designated as three separate Exposure Area DUs (Option 2 in Figure C-23). This option better agreed with the anticipated type of contamination expected in each area, but the risk assessors and remediation experts were still not satisfied with this approach. The risk assessors pointed out that the worker housing unit area as a whole was still too large to treat as a single, Exposure Area DU for future residential redevelopment. They also noted that the Phase I assessment indicated that different neighborhoods of the housing area were developed at different time periods and that contamination within each area might also differ. The remediation experts stated that different types of contamination were also expected between different factory sites and that a better sample data resolution of these areas was also required.
Option 3:
Under Option 3, the former worker housing area was divided into five neighborhoods based on the dates when the buildings were constructed (Option 3 in Figure C-23). Each of these areas as well as the former office building area was further divided into approximately 20,000 ft2 Exposure Area DUs for sample collection (total 36 DUs). This reflected the maximum exposure area size for high-density, residential housing agreed upon by the risk assessors.
Each of the former factory areas were similarly designated as separate Exposure Area DUs (see Figure C-23). The risk assessors felt that that this was adequate for Factory Areas 3, 4 and 5 but felt that the sizes of areas 1 and 2 were still too large to serve as default, exposure areas. The remediation experts pointed out that isolated areas of heavy contamination were known to be present in all factory areas and would likely require expensive remediation. A much higher resolution of contamination in these areas would be required to optimize remediation and manage costs.
Option 4:
Additional exploratory boreholes and test pits were subsequently carried out in each of factory area to help identify areas of heavy contamination and designate Source Area DUs for testing and/or upfront removal. Option 4 in Figure C-23 depicts subdivision and designation of Factory Area FA-2. Lateral and vertical Boundary DUs in anticipated, clean areas were designated along the borders of heavily contaminated areas to isolate soil for remediation. Areas that were not suspected to be heavily contaminated were designated as larger, Exposure Area DUs for testing.
This allowed the entire factory area to be characterized at least at the surficial level, as was the case for the industrial complex as a whole. This only marginally increases field time and cost and helps assure future residents that the property has been thoroughly tested as well as protect the developers from future liability concerns.Both the risk assessors and remediation experts were satisfied with the resolution of the sample data to be generated. Decision Statements regarding risk and the need for remediation were developed for contaminants associated with each designated DU. This helped minimize the need to additional excavation after soil removal.
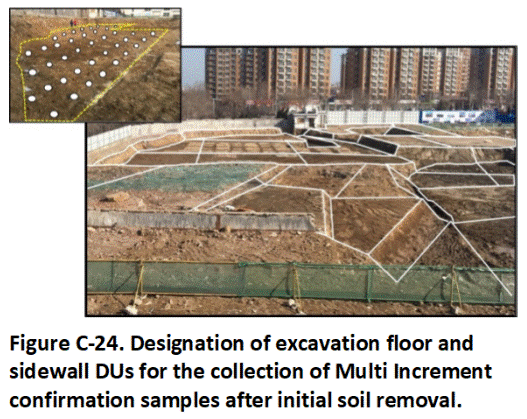
Initial removal and treatment of heavily contaminated soil was carried out without additional sampling of this soil. Fifty-increment, 1 to 3 kg confirmation samples were collected from the floors and sidewalls of the excavations for samples to be tested for non-volatile chemicals (Figure C-24; see Appendix H). Samples to be tested for volatile organic compounds were placed in methanol in the field, with a minimum of 300 grams of soil collected (e.g., 30 ten-gram increments; see Appendix I). Additional soil removal was carried out as dictated by initially collected, subsurface DU data. Source areas treated using in situ techniques were retested for confirmation of cleanup. This well-thought-out, systematic approach allowed the project to be completed in an efficient and predictable manner within the projected deadline and budget.
Return to the Top of the Page
C.12 INVESTIGATION OF PETROLEUM RELEASES
Petroleum is the most common contaminant in the environment. The examples presented below studies focus on the designation of DUs for petroleum releases from oil wells, petroleum fuel pipelines and petroleum fuel terminals. Characterization of subsurface contamination is required in most cases due to seepage of petroleum into the ground or initial releases from buried pipelines and tanks.
The environmental risk posed by petroleum contaminated soil is evaluated in terms of Total Petroleum Hydrocarbons (TPH) and individual, indicator compounds such as benzene, toluene, ethylbenzene, xylenes (BTEX) and targeted polynuclear aromatic hydrocarbons (PAHs). Potential environmental concerns include direct exposure, leaching and contamination of groundwater or surface water, vapor intrusion into existing or future buildings and toxicity to plants and animals. Risks posed by short-term but strong emissions of vapors from heavily contaminated soil disturbed during excavation and redevelopment activities as well as fouling of construction equipment can cause significant cost overruns and delays in projects and must also be addressed. Non-specific, aliphatic and aromatic compounds collectively reported as TPH often pose the greatest risk due to their abundance over individually evaluated chemicals (Brewer et al. 2014; HIDOH 2018a; ITRC 2018).
C.12.1 Crude Oil Pipeline
This example presents a pipeline release of an estimated 1,000,000 liters of light crude oil into a wetland with no public access (Figure C-25). A more detailed discussion of this example is presented in HIDOH 2018a). Oil in upland areas seeped into the soil and became entrapped as isolated pockets and droplets within the capillary fringe of the water table. Oil that spread out across the surface of the wetland had immediate, acute effects on aquatic plants, fish, reptiles, birds and insects caught within the immediate release area as well as benthic organisms in exposed sediment along the marsh edge.

Biodegradation led to the formation of an emulsified mixture of water and degraded petroleum at the surface of the marsh and at the water table. Clumps of degraded oil floating on the surface water drifted to the shoreline and adhered to plants or sank into the underlying sediment.
Source Area DUs were designated in the vicinity of the initial release site based on field observations of heavy contamination (Figure C-26). Additional Ecological Exposure Area DUs were designated by risk assessors for assessment of shallow sediment along the marsh edge and sediment in adjacent, deeper areas of the marsh. The DUs were designated based on the nature of the aquatic habitats and anticipated depositional patterns for droplets of degraded crude oil.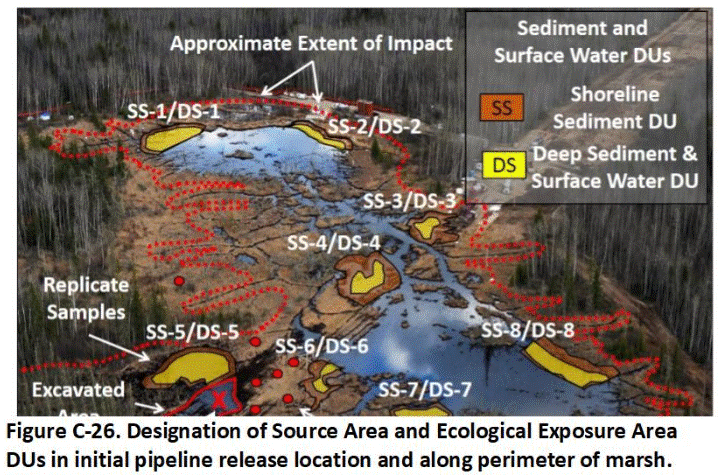
Sediment coring devices were used to collect 30-increment samples in all DUs (refer to examples in Section C.12). The upper 1.5 feet of sediment was targeted for sample collection. Two layers in each DU were designated for testing, the first representing the apparent, biologically active zone of the sediment as indicated by field observations (e.g., 0 to 6″) and the second representing the remainder of the underlying sediment (e.g., 6″ to 18″).
Samples were tested for TPH, BTEX and targeted PAHs. Some samples were also tested for specific aliphatic and aromatic carbon ranges to better predict the toxicity and fate and transport of the residual mixture. TPH data for samples of deeper sediment collected away from the shoreline areas varied widely, with some areas within anticipated background for organic-rich sediment and other areas significantly exceeding screening levels. Replicate data were poor, confirming the presence of small nuggets of tarry, degraded petroleum in the sediment.
Easily accessible, heavily contaminated soil, sediment and vegetation was removed and transported offsite for treatment and disposal without additional testing. Booms and sorbent pads were used to reduce further spread of oil throughout the marsh. The ecological risk assessors determined that additional actions to physically remove contamination would do more harm than benefit to the marsh environment. A slurry wall was constructed around residual contamination in the initial release area to prevent migration of acutely toxic groundwater into marsh. Semi-annual monitoring of surface water, sediment, groundwater and overall health and rebound of marsh ecosystem was to be evaluated for a period of five years and the need for additional, active remediation reviewed at that time.
C.12.2 Active Gas Station
This example presents an active gas station scenario with a history of waste pits and releases from a fuel pipeline and underground storage tanks (USTs) over time. A more detailed discussion of a similar example is presented in HIDOH 2018a.
A detailed characterization of surface and subsurface releases of petroleum, including mean contaminant concentration (and mass) for designated, DU areas and volumes of soil is normally not practical at active gas stations due to the presence of actively used piping and other subsurface infrastructure. In this example, the primary objective of an investigation was to estimate the lateral and vertical extent of obvious heavy (gross) contamination for in situ remediation and/or long-term management. A precise estimation of mean, contaminant concentrations is not necessary. Exploratory borings, pits and trenches were used instead to approximate the extent and relative magnitude of contamination (Figure C-27; refer also to Appendix G). Decisions were to be made based on data for individual cores, rather than data based on the designation of more typical, three-dimensional DU areas and layers.
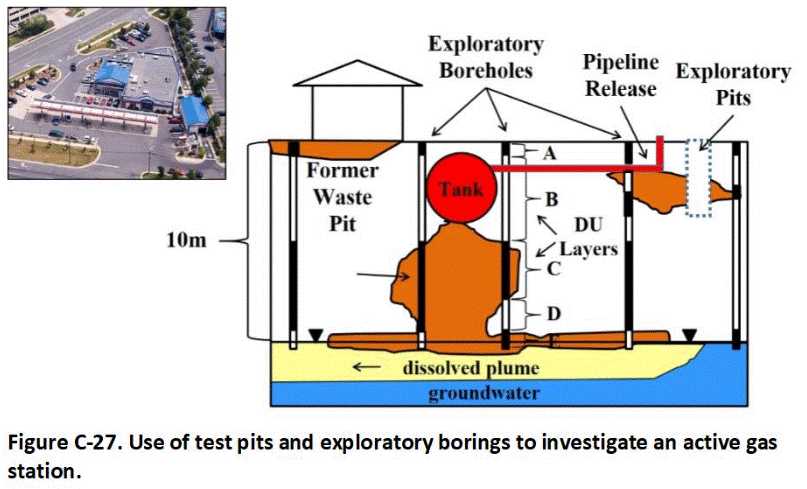
Each core was subdivided into DU intervals for individual testing based on field observations and screening using a photoionization device (PID). Four intervals were typically designated – a thin interval of imported fill material, an upper zone of clean soil, a zone of contaminated soil and a thin interval within the capillary fringe of the water table. Similar DU intervals were designated for borehole cores installed immediately outside of the contaminated area to verify the absence of contamination.
A plunger device was used to collect a minimum 300-gram sample along the entire extent of each targeted DU interval in a boring (refer to Appendix I). Individual samples were placed in methanol in the field and submitted to the laboratory for analysis of TPH, BTEX and naphthalene. Groundwater and soil vapor data were also collected. The results of the investigation were used to design an in situ, soil vapor extraction system.
This approach allowed the entire core to be tested and more reliable data on the extent and nature of subsurface contamination. More detailed DU-MIS data can be collected from key areas of the contamination as needed, for example to better estimate the mass of hydrocarbons present and optimize the in situ soil vapor extraction system.Return to the Top of the Page
C.13 GAS STATION CLOSURE
Closure of a gas station similar to the above example for redevelopment of the property will require removal of the UST system, including tanks, piping and dispensers. In this example, obviously contaminated soil has been excavated and disposed of offsite. Multiple DUs were then designated for confirmation testing (Figure C-28). Each former tank and dispenser location was designated as a separate DU. Decision Units were designated within the former piping area based on the design of individual corridor areas.
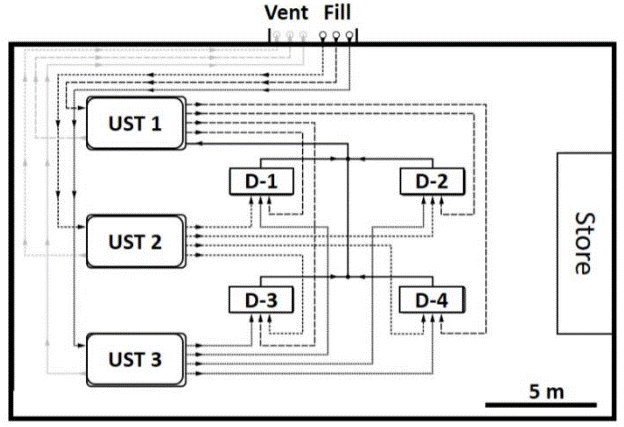
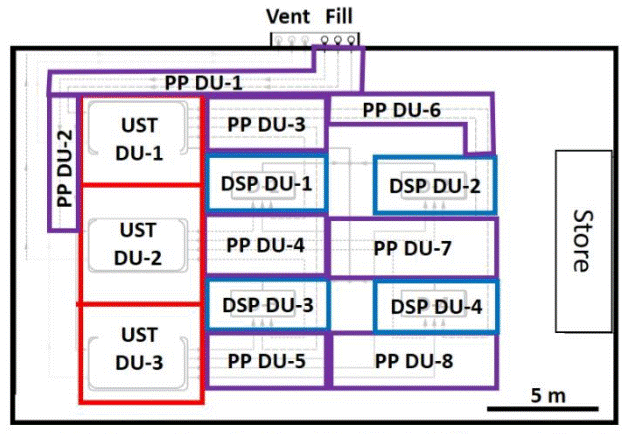
Figure C-28. Designation of UST, piping (PP) and dispenser (DSP) DUs for the confirmation of closure or former gas station.
Two MI samples were collected from the walls and floors of each UST DU, one to be tested for TPH as diesel and one to be tested for volatile TPH as gasoline and BTEX (refer to Appendix I). Two replicate sets of samples were collected from the floor of UST 2 where a leak had been formerly identified and cleaned up.
Excavations for the piping and dispenser areas were relatively shallow (<3 ft). No leaks were identified during removal. A single set of MI samples was collected from the floor of each DU area for confirmation. Two replicate samples were collected from one of each of the piping and dispenser DUs. No further action was determined to be necessary based on the resulting sample data.Return to the Top of the Page
C.14 SEDIMENT DECISION UNITS
The following examples present basics concepts of DU designation for different types of release scenarios and aquatic environments. Refer to Appendix J for a detailed discussion of the collection of sediment samples.
C.14.1 Drainage Canal
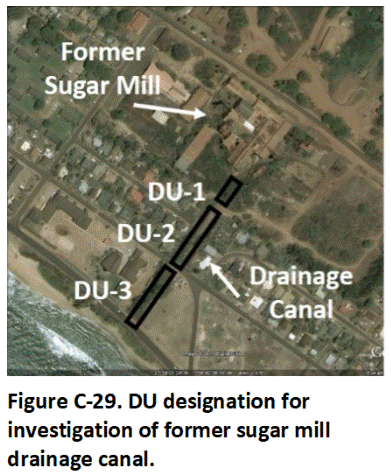 Figure C-29 depicts sediment DUs designated for a shallow drainage canal that once carried wastewater from a sugar mill. Testing of surface soil at discharge points suggested that sediment in the canal might be contaminated with mercury (used as a fungicide). A relatively small DU was designated for the area of the canal immediately downstream of the discharge area (DU-1 in Figure C-29). Two additional and somewhat larger DUs were designated for areas of the canal further downstream (DU-2 and DU-3). Two DU layers were designated, 0 to 15 cm and 15 to 50 cm (0 to 6 and 6 to 20 inches), based on an initial objective to test the upper biologically active layers of sediment and sediment in the canal most likely to become mobilized during flooding events. The deeper sediment was anticipated to be relatively clean.
Figure C-29 depicts sediment DUs designated for a shallow drainage canal that once carried wastewater from a sugar mill. Testing of surface soil at discharge points suggested that sediment in the canal might be contaminated with mercury (used as a fungicide). A relatively small DU was designated for the area of the canal immediately downstream of the discharge area (DU-1 in Figure C-29). Two additional and somewhat larger DUs were designated for areas of the canal further downstream (DU-2 and DU-3). Two DU layers were designated, 0 to 15 cm and 15 to 50 cm (0 to 6 and 6 to 20 inches), based on an initial objective to test the upper biologically active layers of sediment and sediment in the canal most likely to become mobilized during flooding events. The deeper sediment was anticipated to be relatively clean.
DU-1 was 25 ft long and averaged 10 ft wide. DU-2 and DU-3 were 75 ft long and again average 10 ft wide. The in situ volume of sediment in the two layers of DU-1 was approximately 4.7 cyds for the first layer and 11 cyds for the second layer. The volume of sediment in the two layers of DU-2 and DU-3 is estimated to be 14 cyds for the first layer and 28 cyds for the second layer. Both the ecological risk assessors and remediation experts agreed that the resulting data would be useful to assess risk and, if necessary, design a remedial action.
C.14.2 Wastewater Outfall
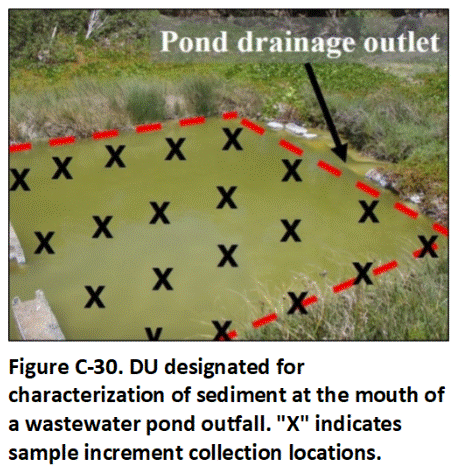 The next example illustrates a single sediment DU designated at the outfall of a wastewater pipe. A single 1000 ft2 DU was designated given the anticipated similarity of impacts within the small area (Figure C-30). Unconsolidated sediment extended to a depth of up to three feet. The full depth of unconsolidated sediment was tested as a single DU (total DU volume 100 cyds). A 30-increment sample was collected using a sampling tube (refer to Appendix J). The sediment was determined to be contaminated with heavy oil, PAHs and lead and subsequently removed for disposal. No confirmation sample was necessary since the entire extent of unconsolidated sediment was tested.
The next example illustrates a single sediment DU designated at the outfall of a wastewater pipe. A single 1000 ft2 DU was designated given the anticipated similarity of impacts within the small area (Figure C-30). Unconsolidated sediment extended to a depth of up to three feet. The full depth of unconsolidated sediment was tested as a single DU (total DU volume 100 cyds). A 30-increment sample was collected using a sampling tube (refer to Appendix J). The sediment was determined to be contaminated with heavy oil, PAHs and lead and subsequently removed for disposal. No confirmation sample was necessary since the entire extent of unconsolidated sediment was tested.
C.14.3 Transformer Spill
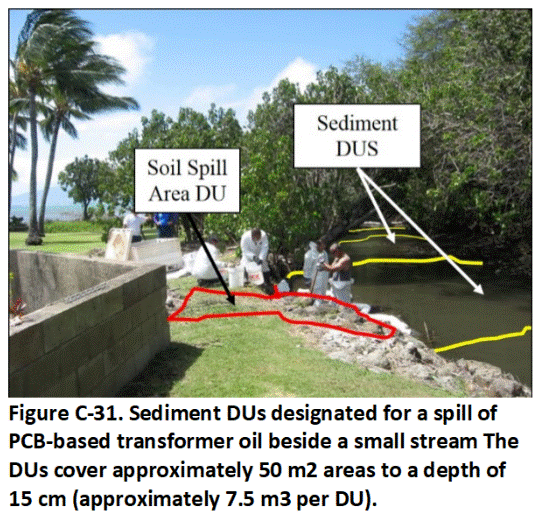 Figure C-31 depicts DUs designated for a PCB spill suspected to have entered a small stream. The area outlined in red depicts the upland area impacted by the spill. A Source Area DU was designated to characterize this area, including Boundary DUs to confirm that the edges of contamination. Relatively small DUs, depicted in yellow, were then designated in the stream itself for characterization of sediment.
Figure C-31 depicts DUs designated for a PCB spill suspected to have entered a small stream. The area outlined in red depicts the upland area impacted by the spill. A Source Area DU was designated to characterize this area, including Boundary DUs to confirm that the edges of contamination. Relatively small DUs, depicted in yellow, were then designated in the stream itself for characterization of sediment.
The location and size of the sediment DUs might be based on stream flow characteristics (e.g., focus on individual depositional areas, including pools and bars) and the maximum volume of sediment to be included within a DU with respect to ecological and remedial considerations. In this example, DUs approximately 500 ft2 in area were considered appropriate. In this example, sediment cover in the stream was very thin, 2 to 4 inches in most areas, and the entire volume of sediment within each DU was targeted for characterization (approximately 2.5 to 10 cyds per DU).
A single 50-increment sample was collected in each DU with triplicate samples collected in the DUs closest to the transformer release area. Contamination above sediment screening levels, intended to be protective of benthic organisms, was not identified.
C.14.4 Pond with Historic Wastewater Discharges
Figure C-32 depicts a much larger sediment investigation carried out in the upper part of a spring-fed 50 acre estuary suspected to have been impacted by historic arsenic-contaminated wastewater and runoff from past agricultural operations in the area. Decision Units were designated based on potential source areas associated with the locations of past facilities as well as areas based on ecological habitats.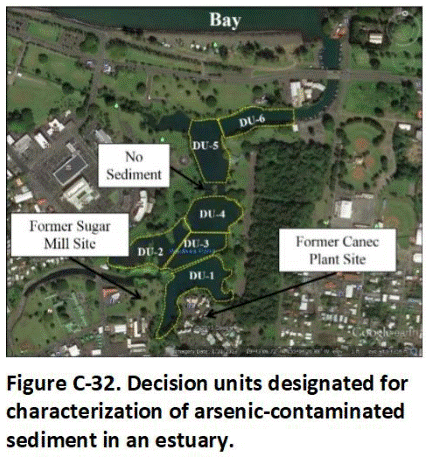
In the example, DU-1 was placed to characterize sediment in the immediate areas of a former sugar mill and a former Canec production facility (used to make arsenic-infused, termite-resistant press-board panels from waste sugarcane fibers.) The remaining DUs reflect sediment areas more distant from the former Canec plant site. The area of the pond encompassed by DUs 2 through 5 are relatively low energy and characterized by fine silts. The lower area of the pond, DU-6, is higher energy due to focused tidal action and characterized by a mix of silts to medium-grained sand. A narrow, high-energy area between DU-4 and DU-5 was not sampled due to the lack of sediment.
Vertical layers for specific DUs were designated based on observations from initial test cores (e.g., distinct layering, grain size, aerobic versus anaerobic zones, etc.), characterization of benthic zones for use in ecological risk assessments, estimated depositional depth since closure of an industrial facility formerly located in the area, and/or desired resolution for potential remedial actions. In the example depicted in Figure C-32, the sediment was divided into three DU Layers for testing (Figure C-33): 1) 0 to 4 in, 2) 4 to 8 in and 3) 8 to 12 inches.
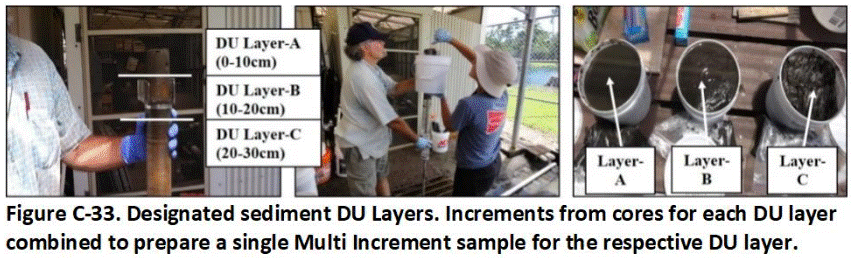
Methods for the collection of MI samples of sediment are reviewed in Appendix J. Thirty-increment samples were collected from each of the DUs and for each DU layer using a small boat and a manual sampling tube. Increments extracted from individual cores were combined between cores to prepare a single sample for each DU layer. Replicate samples were collected from independent cores in the DU closest to the former manufacturing facility.
The final sediment samples in the example were too large for submittal to the laboratory and required subsampling in the field to reduce the sample mass (refer to Appendix J). The results of the investigation identified contamination with arsenic above screening levels in all DUs. Testing of algae, fish and crabs from the pond indicated minimal uptake of arsenic, however, a decision was made not to place restrictions on use of the pond for recreational purposes. Any sediment excavated from the pond in the future was to be disposed of at a regulated landfill.
C.14.5 Harbor Dredging
Testing Requirements
 Refer to Appendix H for guidance on the collection of sediment samples. In this example, dredging is to be carried out at a hypothetical, small boat harbor in a marine environment (Figure C-34). There are three options under initial consideration for disposal and/or reuse of the sediment: 1) Disposal in a pre-established, permitted open water area, 2) Disposal at a regulated, upland landfill, and 3) Reuse as fill material in an upland area.
Refer to Appendix H for guidance on the collection of sediment samples. In this example, dredging is to be carried out at a hypothetical, small boat harbor in a marine environment (Figure C-34). There are three options under initial consideration for disposal and/or reuse of the sediment: 1) Disposal in a pre-established, permitted open water area, 2) Disposal at a regulated, upland landfill, and 3) Reuse as fill material in an upland area.
Characterization of sediment to be disposed of in open water is regulated by the US Army Corps of Engineers and includes consideration of parameters such as particle and toxicity to benthic organisms (USACE 2004). Although somewhat less rigorous than the DU-MIS methods discussed in this guidance, regulator sample collection and testing methods are considered to be adequate for the intended disposal purposes. Option 1 was dismissed, however, due to the lack of a nearby, permitted area and the cost to transport the sediment to a more distant area.
Option 2, disposal at a regulated landfill, was also dismissed due to costs as well as landfill capacity concerns. This left Option 3, reuse of the dredged material as fill material in an upland area as the only acceptable alternative. Proposals for disposal or reuse dredged sediment in an upland area requires consultation with the regulatory agency that oversees the reuse of recycled “soil” (e.g., Hawai’i Department of Health, HIDOH Solid and Hazardous Waste Branch). Characterization must be carried out using DU-MIS methods described in this guidance document.
DU Designation
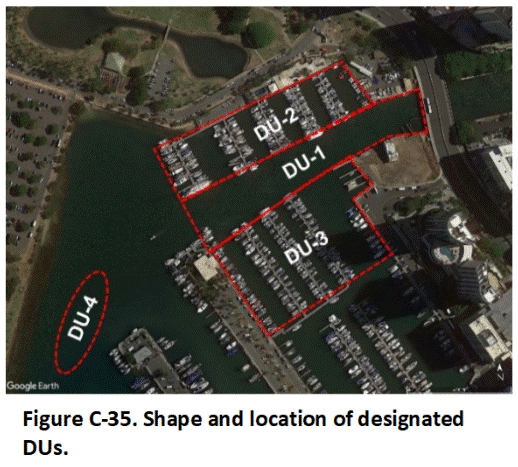 Four areas are targeted for dredging, with each area designated as a separate DU (Figure C-35). The DUs were designated only for assessment of reuse in upland areas. Assessment of potential ecological impacts of contaminants in the sediment on benthic or other aquatic organisms was not within the scope of work for the project.
Four areas are targeted for dredging, with each area designated as a separate DU (Figure C-35). The DUs were designated only for assessment of reuse in upland areas. Assessment of potential ecological impacts of contaminants in the sediment on benthic or other aquatic organisms was not within the scope of work for the project.
A dredge depth of five feet is planned for the main channel area designated as DU-1. A dredge depth of three feet is planned for DU areas 2 and 3 in the adjacent boat slip areas. The current average water depth of the channel is five feet.
The average water depth of the slip areas is three to four feet. Preliminary drilling indicated that the upper three to five feet of sediment in these areas is characterized by unconsolidated silty clay to clayey silt. Dredging to a depth of five feet is required for the DU 4 area in order to remove a medium- to coarse-grained sand bar. The water depth in this area ranges from six to eight feet.
A total in situ dredge volume of approximately 84,200 cubic yards (cyds) of sediment was anticipated. Sediment volume following dewatering and natural compaction was predicted to be approximately 75% of the in situ estimate for clayey silt for an adjusted final volume of 65,000 cyds.
Relatively large DU areas were considered acceptable based on assumed, relatively uniform, lateral distribution of any contaminants present within the sediment. The DU areas were divided into two, 1.5-foot-thick upper layers and, in the case of DU-1, a third, lowermost two-foot-thick layer in order to consider possible higher concentrations of contaminants in deeper layers of sediment (e.g., due to lead from earlier auto exhaust). Depth intervals of this thickness (at least 1.5 feet) also correlate well to the anticipated dredge method (barge-mounted excavator), where thinner layers would be difficult to discern during the dredge process should any of the layers be found to contain contamination.
Testing of material in the sand bar designated as DU-4 was not required by the overseeing regulatory agency due to the lack of fines more prone to hold contaminants. A similar division of the sand into three DU layers for sample collection was determined appropriate by the responsible party, in this case the county government, for due diligence purposes.
The final area and approximate, in situ and ex situ volume of sediment in each DU is summarized in the below table:
Final DU Designation.
| Primary DU Area | Final DU ID Number | Sediment Type | Area | Sediment Interval | Estimated In Situ Sediment Volume | *Estimated In Situ Sediment Volume | ||||||||||||
| DU-1 | DU-1A | Clayey Silt | 175,000 ft2 | 0.0 – 1.5 ft | 9,700 cyds | 7,300 cyds | ||||||||||||
| DU-1B | Clayey Silt | 175,000 ft2 | 1.5 – 3.0 ft | 9,700 cyds | 7,300 cyds | |||||||||||||
| DU-1C | Clayey Silt | 175,000 ft2 | 3.0 – 5.0 ft | 13,000 cyds | 9,700 cyds | |||||||||||||
| DU-2 | DU-2A | Clayey Silt | 150,000 ft2 | 0.0 -1.5 ft | 8,300 cyds | 6,250 cyds | ||||||||||||
| DU-2B | Clayey Silt | 150,000 ft2 | 1.5 – 3.0 ft | 8,300 cyds | 6,250 cyds | |||||||||||||
| DU-3 | DU-3A | Clayey Silt | 250,000 ft2 | 0.0 – 1.5 ft | 13,900 cyds | 10,400 cyds | ||||||||||||
| DU-3B | Clayey Silt | 250,000 ft2 | 1.5 – 3.0 ft | 13,900 cyds | 10,400 cyds | |||||||||||||
| DU-4 | DU-4A | Sand | 40,000 ft2 | 0.0 -1.5 ft | 2,200 cyds | 2,200 cyds | ||||||||||||
| DU-4B | Sand | 40,000 ft2 | 1.5 – 3.0 ft | 2,200 cyds | 2,200 cyds | DU-4C | Sand | 40,000 ft2 | 3.0 – 5.0 ft | 3,000 cyds | 3,000 cyds | Total: | 84,200 yds | 65,000 cyds | ||||
*Assumes minimum 75% reduction of original in situ volume of clayey silt material following dewatering; rounded.
The <2mm fraction of sediment was designated as the targeted DU particle size, corresponding to requirements for testing of upland soil by the overseeing regulatory agency. Stormwater runoff is considered to be the primary source of any potential contamination. No boat maintenance areas, open dumping of waste or other past or current point source areas of contamination are known to be present.
Targeted Contaminants of Potential Concern
Potential contaminants of concern include organochlorine pesticides, lead, PCBs, heavy petroleum and PAHs. Salinity is also considered a contaminant of concern for proposed upland placement of the sediment due its location in a marine environment and concerns regarding potential toxicity to upland plants. Phytotoxicity is assessed based on testing of Electrical Conductivity and Sodium Absorption Ratio data (HIDOH 2017). An Electrical Conductivity greater than 2 mS/cm and/or a Sodium Absorption Ratio greater than 5 is assumed to pose potential phytotoxicity concerns for typical, upland plants.
Sample data for targeted contaminants of potential concern will be directly compared to comprehensive, Environmental Action Levels for direct exposure, leaching and gross contamination concerns published by the overseeing regulatory agency (e.g., refer to HIDOH 2017). Appropriate restrictions will be placed on reuse of the dredged material based on the results of this comparison and in absence of a more detailed, case-specific environmental risk assessment.
In Situ vs Ex Situ Sample Collection
The collection of 30-increment samples was approved based on the assumed water-based deposition of fine, particulate material and lack of large particles and fragments of waste material in the sediment. Options for characterization include: 1) Collection of samples ex situ after placement of the sediment in stockpiles to dewater, following stockpile sampling methods (refer to Appendix H) or 2) In situ collection of samples prior to dredging. The option for in situ sample collection was selected due to permitting and site preparation costs and requirements for an interim staging area and a desire to complete the project as quickly as possible.
Two independent sets of thirty replicate cores were designated to be collected from DU-1 in order to test the precision of the overall sampling and analysis methods (“Field Replicates”). The terms (FR1) and (FR2) were added to end of the DU Layer identification numbers in order to identify the replicate samples.
Sample Collection Methods
Vibracore equipment was determined to be the most appropriate sampling method given the need to collect increment cores from a boat and the depth of the water involved (Figure C-36). Use of a Mini Vibracore was determined to be adequate for testing of DUs 1, 2 and 3 in the shallower and less turbulent areas of the harbor. A standard, more heavy duty Vibracore was selected for the collection of increment cores from DU-4. This was due both to multiple factors, including the depth of the water, a potential for stronger currents, the depth of coring needed and the presence of relatively dense sand in the bottom five feet of the DU.
Core increments were collected in a systematic random manner in each of the DUs. A GPS device was used to move the boat to each collection point (see Figure C-36). The Vibracore was lowered to the top of the sediment at each location using a weighted platform provided by the supplier. A five-foot, two-inch diameter acetate and/or aluminum sampling tube with an inner liner was used to collect increments in DUs 1,2 and 3 with the mini Vibracore. A ten-foot, four-inch diameter tube with a liner was used to collect increments from DU-4 with the full-size Vibracore.
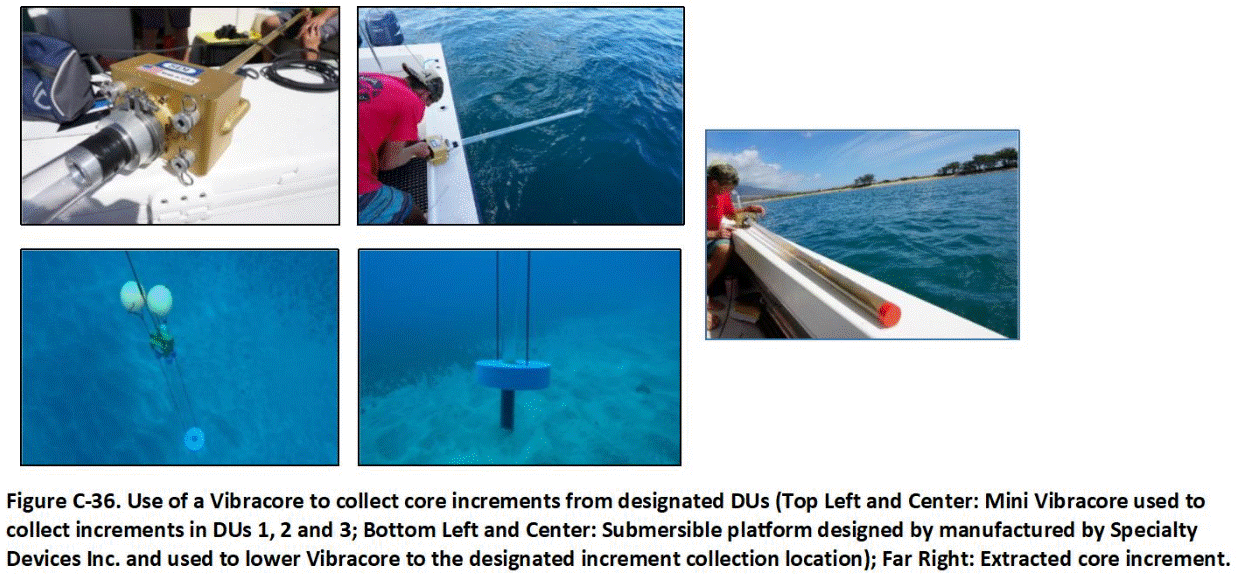 A sediment-catch device was attached to the bottom of each core barrel in order to minimize loss of material during retrieval of the barrel to the surface. Triplicate sets of increment cores were collected from independent areas of DU-1 using the equilateral triangle spacing approach depicted in Figure L-2 of Appendix L.
A sediment-catch device was attached to the bottom of each core barrel in order to minimize loss of material during retrieval of the barrel to the surface. Triplicate sets of increment cores were collected from independent areas of DU-1 using the equilateral triangle spacing approach depicted in Figure L-2 of Appendix L.
Sample Preparation
Increment cores were opened for the collection of subsamples immediately following retrieval at each location. Sediment was observed to have compacted during insertion of the core barrel in many of the samples extracted from DUs 1, 2 and 3. The midpoint of the core was assumed to represent the original contact between DU Layers A and B (i.e., original depth of 1.5 feet).
The volume of sediment in the cores was too large for combination of complete increments into a single sample. As an alternative, an approximately 100-gram subsample was collected from the interval in a systematic random method using a plastic syringe-type sampling device (refer to Appendix J). Increment subsamples corresponding to a targeted layer for the specified DU were combined across cores and placed into a new, heavy-duty LDP freezer bad for preparation of a bulk sample. The total mass of each sample was approximately three kilograms. Subsample collection and sample preparation was carried out in a similar manner for core increment collected from DU-4. No compaction of the sand interval was indicated in the core.
Replicate subsamples were collected from each of the DU-1 layers for followup assessment of the precision of the field subsampling method (“Field Subsampling Replicates”). Overall retrieval of the targeted core intervals was adequate for most cores. Poor retrieval of sediment in a small number of cores was not considered to have significantly biased the resulting bulk sample due to the relatively large number of increments collected in each DU.
A total of 21 samples were prepared for analysis during the investigation – 10 primary samples, six field replicate samples and four field subsampling replicates. Samples were immediately placed on ice and shipped to the laboratory for further processing and testing the following day. Excess sediment was placed back within the DU area where the corresponding sample increments were originally collected.
Laboratory Processing
Each sample was spread into a 1cm-thick (0.4 in) layer on a dedicated tray and air dried at 25°C for approximately 72 hours following receipt by the laboratory. Air drying at room temperature was considered to be acceptable to the nonvolatile nature of the targeted contaminants and anticipated minimal degradation during this time period.
Following drying, samples were crushed using a mortar and pestle in order to break up clumps of dried clay and silt. Samples were then passed through a #10 sieve and >2mm-size particles removed. A sectoral splitter was then used to prepare an adequate number of ten-gram subsamples for testing. The collection and testing of replicate subsamples to assess subsample collection precision (“Laboratory Subsampling Replicates”) was determined to be unnecessary due to use of the sectoral splitter.
Results
Concentrations of all targeted contaminants were well below action levels set by the regulatory agency for unrestricted reuse of the dredged material. The salinity of the sediment, however, significantly exceeded action levels for potential phytotoxicity to upland plants (salinity similar to fast-food French fries). The clayey nature of sediment from all but the sandy interval of DU-4 further precluded use of the material for structural fill beneath buildings or roadways. Approval was made to reuse the silty and clayey sediment as fill material in low lying areas of the adjacent coastal park without further testing. Sand would be used for beach replenishment at the park. Dredged sediment is to be stockpiled on site and dewatered. The sediment will then be placed in the selected areas and amended as needed to promote the growth of salt-tolerant plants.
APPENDIX D. SAMPLING THEORY AND MULTI INCREMENT SAMPLE COLLECTIONReturn to the Top of the Page
D.1 THEORY OF SAMPLING
D.1.1 Early Mineral Exploration Industry
Sampling theory for testing of particulate matter such as soil and sediment was developed by the mineral exploration industry in the 1800s and 1900s. Mineral exploration involves detailed mapping to identify potentially valuable bodies of mineral bearing ore. This is much like a Phase I investigation in the environmental industry, where existing information is used to identify potential areas of contamination. When a promising ore body is found, multiple cores are collected and tested. The resulting sample data are used to make an initial estimation of the mean concentration of the commodity in the ore body as a whole, referred to as the “grade” of the ore. This is then used to estimate the total mass of the commodity present. If the estimated mass of the commodity present in the ore meets minimum requirements for profitable exploitation, then the ore is excavated, crushed into small fragments, retested to verify grade, and in most cases sold to a third party for further processing and extraction of the targeted commodity (Figure D-1).
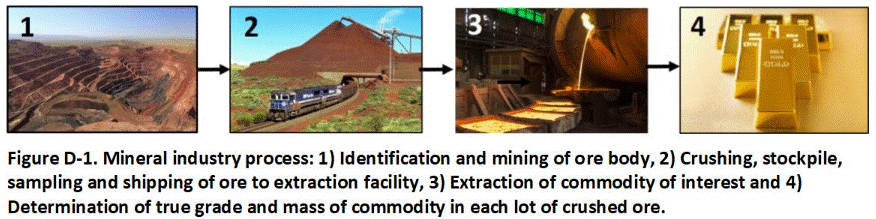
The true mass of a commodity in a specified volume of processed ore can only be known after the ore is extracted. Accurate estimation of commodity mass requires an equally accurate collection of representative sample data. Poor sampling methods can lead to significant over estimation or, more commonly, under estimation of commodity mass. This was indeed the case until relatively recently in the mining industry. Estimates of the mean concentration of a commodity for a targeted body of processed ore based on statistical analysis of individual, “discrete” sample data or “composite” sample data often proved to be highly inaccurate. Bankruptcies and lawsuits were common, as were missed opportunities for profitable mining and use of much needed but overlooked, natural resources.
D.1.2 Development of Gy’s Theory of Sampling
Background
Beginning in the late 1800s, mining specialists began working with statisticians to understand the causes of poor data quality and develop more reliable methods of sample collection. The result was compiled and summarized by Pierre Gy in the 1950s and is now referred to as the Gy’s Theory of Sampling (Pitard 2005, 2009, 2019; Ramsey and Hewitt 2005; Esbensen 2020).
Gy’s Theory of Sampling and subsequent expansions of the approach were developed to address two deceptively simple questions: 1) “What is the minimum sample mass required to represent a targeted volume of crushed ore within a specified level of confidence?” and 2) “How should the sample be collected in the field?” and 3) How should the sample be processed and analyzed at the laboratory? Answering these questions required a detailed understanding of the sources of error in sampling data. Over several decades, error was systematically categorized and evaluated in terms of the individual stages of the data collection process:
- Designation of area and volume of material for testing;
- Collection of sample in the field;
- Processing of sample at the laboratory;
- Collection of a subsample for analytical testing; and
- Analytical testing.
The term “lot” is used in the mining industry for a specified volume of crushed ore that is to be sampled and tested for a targeted commodity of interest, such as gold or iron. The concept of “lots” is identical to the concept of DUs in the environmental industry as well as other sampling specific industries (AFFCO 2015; refer to Appendix C). Lots are designated for testing to assess the overall grade and economic viability of an ore deposit or to optimize mining and mineral extraction processes.
The quality of the samples collected is controlled by steps taken in the subsequent stages of the project. Mining experts quickly realized that analytical testing of a collected subsample introduced the least error into the data, due to strict test method protocols and requirements for analytical quality assessment and control at the laboratory. The primary source of data unreliability was determined to be related to four factors: 1) Collection of the representative sample in the field; 2) Processing of the sample at the laboratory; 3) Collection of a representative subsample for testing and 4) Analysis of the subsample. The greatest error was determined to be associated with the collection of a representative sample in the field followed by processing and subsampling of the material at the laboratory. Error associated with analysis of the subsample was determined to be in most cases minimal.
This error was directly tied to the inevitable, heterogeneous nature of crushed ore at both the scale of an individual particle and within the targeted lot of material as a whole. Sampling statisticians in the mining industry determined that data quality was controlled by three primary factors: 1) The mass of the sample collected in the field and the subsample tested at the laboratory, 2) The number of points from which the sample was collected and prepared, referred to as “increments”; and 3) The method used to collect the individual increments. The role that each factor played in sampling error and steps to address this error were systematically investigated and ultimately published as Gy’s Theory of Sampling (Pitard 2019).
Compositional and Distributional Heterogeneity
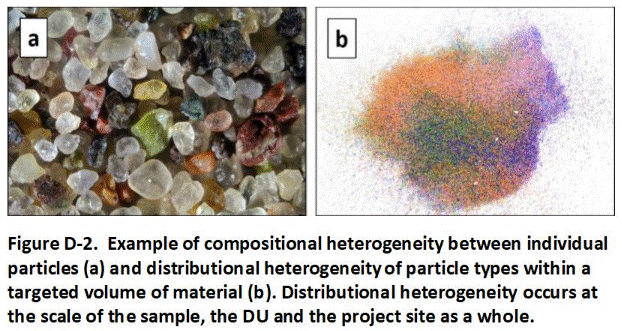 Error associated with collection of a sample in the field and processing and subsampling of the sample at the laboratory is tied to the distributional and compositional heterogeneity of the targeted contaminant in the media (Figure D-2). Compositional heterogeneity (Figure D-2a) refers to variability between individual particles and is described in terms of particle size, shape and density. Other characteristics of importance include whether the commodity of interest is bound up within individual particles or fully liberated and present as individual nuggets.
Error associated with collection of a sample in the field and processing and subsampling of the sample at the laboratory is tied to the distributional and compositional heterogeneity of the targeted contaminant in the media (Figure D-2). Compositional heterogeneity (Figure D-2a) refers to variability between individual particles and is described in terms of particle size, shape and density. Other characteristics of importance include whether the commodity of interest is bound up within individual particles or fully liberated and present as individual nuggets.
Distributional heterogeneity in the mining industry refers to variability of the mean concentration of the commodity within the project area, within the lot to be characterized and even within the sample collected and submitted for testing (refer to Figure D-2b). Mining statisticians realized that a sample collected from a targeted lot of crushed must accurately capture and represent the inherent distributional heterogeneity to obtain data representative of the crushed ore as a whole.
Sample error associated with compositional and distributional error can never be fully eliminated, but it can be controlled and minimized. Error associated with compositional heterogeneity is controlled by collecting an adequate mass of the material. Error associated with distributional heterogeneity is controlled by collecting the sample from a large number of points within the targeted volume of material.
Fundamental Error and Minimum Sample and Subsample Mass
Gy reviewed decades of data for testing of crushed ore and developed an equation to quantify what he referred to as Fundamental Error that relates the error induced by compositional heterogeneity to total sample mass. Although several approaches for quantification of Fundamental Error have since been derived, “FE” is most commonly expressed as (Minnitt 2007):
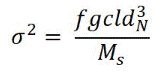
where “σ2” is variance or error, “f,” “g,” “c,” and “l” represent “Shape,” “Granulometric,” “Mineralogical Composition,” and “Liberation” factors that quantitatively describe the variability of particle characteristics, “dN” is the nominal maximum particle size and Ms is sample mass. Particle characteristics can often be estimated and combined as a single constant “K” for specific types of ore and the equation simplified as:
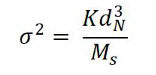
The maximum size of the particles present in the crushed ore was determined to have the greatest influence on sample data error. Fundamental Error could therefore be most efficiently controlled by thorough crushing of the ore and/or the collection of a larger sample mass.
Rearranging the equation to solve for sample mass allows for the minimum mass of a sample required to control Fundamental Error at a specified particle size and confidence level to be calculated:
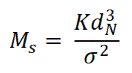
In general, sample collection, processing and testing should be carried out in a manner that reduces Fundamental error to a minimum of 15% (see USEPA 2003b, Minnitt 2007). The mass of a sample required to meet the specified level of Fundamental Error is directly proportional to the cube of the particle size. Comparably small decreases in the maximum particle size by crushing and grinding could therefore lead to substantial decreases in the mass required to address Fundamental Error.
This solved one aspect of the minimum sample mass required to represent a targeted lot of crushed ore. Gy’s research demonstrated that relatively small sample masses could, in theory, be representative of very large volumes of material provided that the ore was crushed to a small enough particle size. For example, a sample mass of only a few grams is predicted to reduce Fundamental Error to the target goal of <15% for material that has a maximum dimension of less than 2 mm.
The only remaining factor in the collection of a representative sample was, in theory, the number of increments to be included in the sample. Research, however, would quickly indicate that the actual collection of increments in the field was itself a large source of data error. Addressing this error would require the collection of much larger masses of crushed ore to obtain reliably representative samples.
Distributional Heterogeneity and Increment Collection Points
Potential sampling errors associated with distributional heterogeneity, collectively described by Gy as Grouping and Segregation Errors (Minnitt 2007; Pitard 2019; Esbensen 2020), are addressed by the collection and combination of a large number of small masses of particles throughout the targeted DU using a systematic-random approach (refer to Appendix D). Each mass is referred to as an “increment.” The final sample prepared is referred to by some researchers as a Multi Increment (MI) sample.
Determination of the number of increment collection locations (or times, in the material is moving by on a conveyor belt) necessary to represent a lot of crushed ore is specific to the nature of the ore being tested as well as the nature of the mining operation and ore processing. In the mining industry, careful studies carried out to compare sample data for a lot of ore based on different numbers of increments to the mass of the commodity determined after extraction. If the nature of the ore changes, then fewer or more increments might be required to collect representative samples. This requires constant coordination with workers excavating ore from the mine as well as workers processing and moving ore within the mining operation.
Controlling Sample Increment Collection Error
All of the particles in the targeted media must be given an equal opportunity of being selected for inclusion for the sample to be reliably representative. If not, then the sample is said to be “biased” (Pitard 2005, 2009, 2019; Minnitt 2007; Esbensen 2020). Bias can in theory be completely eliminated with proper sampling methods. In practice, some error in sample collection is normally unavoidable.
Methods to reduce bias to a negligible level include ensuring that increments are of equal mass and that the shape of an increment is such that it equally represents the full depth interval of the media. For example, a core-shaped increment extracted from the full thickness of a tablet-shaped DU is unbiased both vertically and laterally, while a wedge-shaped increment wider at the top than the bottom can be biased in both dimensions. The physical extraction of each increment is also important. Tools used to collect increments and prepare samples must be suited to the site-specific conditions.Unlike Fundamental Error, error associated with the physical collection of a sample can only be quantified by complete extraction of the targeted commodity of interest and comparison of the resulting mass to the mass predicted by the sample data. This requires extensive training and experience on the part of the sample collection team and preliminary collection and testing of samples using different approaches and tools. This is normally carried out on a project-specific basis and the results used to establish a standard testing procedure for subject ore.
Very stringent control of data error is required in the mining industry due to the economic implications of significant over or under estimation of the total mass of a commodity in a specific volume/mass of crushed ore to be sold on the open market. A relative standard deviation for replicate samples of <5% is often used as a criterion for acceptance or rejection of a lot of crushed ore sold on the open market. The mass of a sample required to address both Fundamental Error and error associated with the physical collection of the sample in the field can range from hundreds to even thousands of kilograms.
Controlling Laboratory Processing and Testing Error
Extensive processing of the primary sample is normally required to prepare a representative subsample of a mass sufficient to meet test method limitations – typically only a few grams (Pitard 2005, 2009, 2019; Esbensen 2020). This is accomplished by following similar methods used to collect the sample in the field. Further crushing of the primary sample to reduce the maximum particle size is normally carried out. This allows the collection of a smaller subsample that can be further ground and crushed into a fine powder. In some cases, samples are melted and turned to glass before crushing to further minimize heterogeneity and improve sample data.
Evaluating Data Usability
The overall reliability and usability of the sample data collected for crushed ore is evaluated by reviewing the methods used to collect, process and test the samples and the precision of replicate sample data (refer to Section 3.9 and Appendix L). If the samples were not properly collected in the field, then the precision of the replicate sample data is irrelevant.
The precision of the sampling method is evaluated by a comparison of data for replicate samples that are collected, processed and tested in an identical manner from the same lot. If the variability of the replicate data is determined to meet quality control requirements, then the sampling method is approved. If not, then an evaluation of quality control for the analytical method employed and data for concurrent testing of replicate, laboratory subsamples is used to determine if the main source of error is associated with the laboratory or with the collection of samples in the field.
Mining experts determined that the main source of error in data was associated with the physical collection of the sample in the field. This was followed by processing of the sample at the laboratory and collection of a subsample for analysis. Analytical error associated with testing of subsamples was determined to be a relatively minor source of error in most cases. If data for laboratory replicate subsamples collected from the same sample were reasonably similar, then error could normally be attributed to the method used to collect the samples in the field. If the laboratory replicate data for individual samples were not in agreement, then the sample processing and subsampling methods were reviewed and adjusted until data variability was reduced to an acceptable level. If unacceptable variability between data for field replicate samples persisted, then the method used to collect samples was reviewed and adjusted. This typically include the need to prepare larger-mass samples from a greater number of increments.
The systematic approach described above for testing of crushed ore dramatically reduced sample data error in the mineral exploration and extraction industry. With only a few modifications, the same approaches can be used to improve data quality and reliability in the environmental industry.Return to the Top of the Page
D.2 APPLICATION TO ENVIRONMENTAL SAMPLING
Sample collection methods developed by the minerals industry and summarized in Gy’s Theory of Sampling apply to environmental testing of soil and sediment as well as to other media made up of an essentially infinite number of small particles (AFFCO 2015). Although the project objectives and economic incentives are different, the systematic approach used to reliably estimate the mean concentration of a contaminant in a designated DU area and volume of soil or sediment is essentially identical to the approach used to estimate the mean concentration (grade) of a commodity such as gold in a designated lot of crushed ore (Table D-1).
Table D-1. Comparison of investigation objectives and methods in the mining versus environmental industries.
| Factors | Mining Industry | Environment Industry |
| Investigation Target: | Commodities | Contaminants |
| Example Media: | Crushed Ore | Soil and Sediment |
| Field Characterization: | Designation of Lots | Designation of Decision Units (DUs) |
| Basis: |
|
|
| Sample Collection: | Multi Increment Samples | Multi Increment Samples |
| 1Typical Sample Mass: | 100s to 1,000 kilograms | 1 to 3 kilograms |
| 2Quality Control: |
|
|
| 3Required Replicate Data Precision: | RSD <1-5% | RSD <35% |
Notes:
1. Minimum 300 grams collected for samples to be tested for VOCs.
2. Includes matrix spikes, replicate analysis of same subsample, etc.
3. Relative Standard Deviation of replicate samples. Higher RSDs might be acceptable for both industries depending on the acceptable margin of error for decision making and hedge or safety factors built into decision criteria.
A higher tolerance for data error is normally acceptable in the environmental industry, where order-of-magnitude or large margins of safety are normally built into screening levels and risk assessments. As discussed in Appendix L, a Relative Standard Deviation of 35% and even higher is normally acceptable for field replicate sample data (15% for laboratory subsample replicate data). This allows for the collection of much smaller samples in the field, typically just 1 to 3 kilograms. As discussed in Section 3 of the TGM, the recommended approach for the risk-based investigation of contaminated soil can be summarized in nine, systematic steps (refer also to noted appendices):
- Step 1: Define investigation scope and establish preliminary Conceptual Site Model (Appendix B);
- Step 2: Identify potentially hazardous chemicals to be targeted for testing (Appendix B);
- Step 3: Determine data information needs in terms of nature of risks associated specific chemicals and/or optimization of anticipated remedial actions (Appendix B);
- Step 4: Designate well-thought-out Decision Unit areas and volumes of soil for testing that will provide the data necessary to meet the stated information needs (Appendix C);
- Step 5: Specify Decision Statements for each DU that describe the action to be taken upon receipt of sample data (Appendices B and C);
- Step 6: Develop and implement the sample collection and analysis plan, including the collection of a Multi Increment Sample from each DU in the field and processing of samples at the laboratory (Appendices D, F, G, H, I, J, K);
- Step 7: Assess data quality based on a review of the sample collection and analysis methods implemented and an evaluation of replicate field and laboratory data to test overall sampling method precision (Appendix L); and
- Step 8: Determine potential environmental hazards based on data for each DU and the review of overall data quality (Appendix B);
- Step 9: Update the Conceptual Site Model and propose additional actions (Appendices B and L).
Development of a similar, nine-step, systematic process for the reliable investigation and characterization of commodity ores only requires modification of the intent of the investigation and according modification of the objectives of the investigation, the basis of DUs and the decisions to be made upon receipt of the sample data.
APPENDIX E. USE AND MISUSE OF DISCRETE SAMPLE DATA
Return to the Top of the Page
E.1 BACKGROUND AND KEY ASSUMPTIONS
A review of the origin of “discrete” or “grab” sampling methods for the investigation of contaminated soil and sediment is provided in Brewer et al. (2017a, b); refer to supplemental information included with paper). A “discrete sample” refers to the collection of a small mass of soil, typically 100 to 200 g, from a single point within an area targeted for investigation. At the laboratory, a random 1 g to 10 g subsample is then collected from the original sample and tested for contaminants of concern. Discrete samples are not routinely processed in a manner than ensures the resulting data are representative of the sample provided as a whole. An attempt might be made to “homogenize” a sample by simple stirring, but this can cause fine and potentially more contaminated particles to settle to the bottom of the container and bias data.
The collection of discrete samples played an important role in site investigation guidance published by the United States Environmental Protection Agency (USEPA) in the 1980s (USEPA 1985, 1986; Gilbert 1987). The Toxic Substances Control Act (TSCA) specifically requires the use of discrete samples for final, decision making purposes. A discussion of the use of DU-MIS methods under TSCA is provided in Appendix N. These guidance documents and regulations were designed to identify and characterize localized areas or “hot spots” of contaminated soil. This reflects the current concept of “Source Area” DUs discussed in Section 3.3.2 and Appendix C. Individual sample points were to be spaced at a distance less than the width or length of the targeted source area size of interest.
Environmental experts at the time were most familiar with the collection of discrete/grab samples to test industrial waste. The concentration of chemicals in industrial waste, especially liquid waste, are normally very consistent as long as the process itself does not change. Under these conditions, a grab sample of limited mass from a random point within a targeted waste stream can be assumed to represent the larger-scale waste stream as a whole reasonably well.The preparers of USEPA sampling guidance similarly assumed that the concentrations of a chemical in soil contaminated by a release of industrial waste would likewise be relatively uniform (notations added):
- “The implicit assumption (in the use of grids of discrete soil samples) that residual contamination is equally likely to be present anywhere within the sampling area is reasonable (USEPA 1985; refer also to USEPA 1986).”
- “The (discrete soil sampling) method makes use of prior information to divide the target population into subgroups (i.e., DUs) that are internally homogeneous (Gilbert 1987).”
- “Any sample located within the contaminated zone will identify the contamination (USEPA 1987).”
- “When there is little distance between points it is expected that there will be little variability between points (USEPA 1989b).”
These assumptions greatly simplified the preparation of guidance for the investigation of contaminated soil, since data for single samples could be used to determine if soil in the immediate area of the sample collection point was contaminated above levels of potential concern. Surprisingly, however, the hypothesized relative uniformity of contaminants within a source area was never thoroughly tested in the field Brewer et al. (2017b).
The guidance also reflects a mistaken belief that risk-based screening levels for soil that were being developed at the time applied to any testable mass of soil. As discussed in concurrent USEPA guidance, risk is instead assessed based on the mean concentration of the contaminant for a specified “exposure area” and by default “exposure volume” of soil as a whole, such as a school playground or an area of exposed soil frequently contacted by workers (USEPA 1987, 1988c, 1989b, c, e, f, 1991g, 1992c, 2014f; refer to Section 3.3 and Appendices B and C). The concentration of the contaminant associated with any given small mass of soil within this area is irrelevant in terms or assessment of risk.
This is true for assessment of both chronic risk, the basis of most screening levels, as well as hypothetical acute risk (refer to Brewer et al. 2017b). Concerns about “dilution” of contaminant concentrations and underestimation of risk based on “composite” sample data and the need to test individual, discrete points were unfounded. Indeed, all concentrations reported by the laboratory represent a “diluted” average of the concentration associated with the collection of individual particles tested. The only question is the designation of a DU of appropriate size to address the nature of the risk question (refer to main guidance and Appendix C).Return to the Top of the Page
E.2 FIELD STUDY OF DISCRETE SAMPLE DATA RELIABILITY
A detailed field study of discrete sample variability and reliability was carried out by the State of Hawaii in 2014 and 2015 (HIDOH 2015,b; Brewer et al. 2017a,b). The research was funded through grants provided by the USEPA.
Three sites ranging in area from 1500 ft2 to 6000 ft2 and known to be contaminated were selected for the study. Hundreds of discrete samples were collected and tested from each site. The first site was contaminated by long-term releases of arsenic-contaminated wastewater. The second site was contaminated by mixing lead-contaminated incinerator ash in soil used as fill material. The third site was contaminated by releases of electrical equipment oil that contained polychlorinated biphenyls (PCBs). The upper 6 inches of soil was targeted for testing.
Twenty-four grid points were established at each study site. Variability of contaminant concentrations between closely spaced samples, referred to as “inter-sample variability,” was evaluated by independent testing of five samples collected within a three-foot by three-foot square area at each grid point. Variability of contaminant concentrations within individual samples, referred to as “intra-sample variability”, was evaluated by testing ten separate subsamples of soil, one of the samples collected at each point. The combined variability between co-located samples and within individual samples reflects the total random variability of discrete sample data that might be expected around a single collection point in the field.
The results of the study are summarized in Figure E-1. The study data demonstrated that the concentration of a contaminant reported by a laboratory for a given discrete soil sample is random within a range of possibilities for the individual sampling point. The magnitude of variability depends on both the type and nature of the chemical released. The predicted variability between co-located samples at the arsenic site was comparatively low, with an average two-fold difference between the maximum and minimum concentration of arsenic in samples collected around a single point. Average variability between co-located samples at the lead-contaminated site was predicted to be seven-fold. The predicted variability between co-located samples at the PCB site was the highest, with an average difference of 39-fold and in some cases greater than two orders of magnitude.
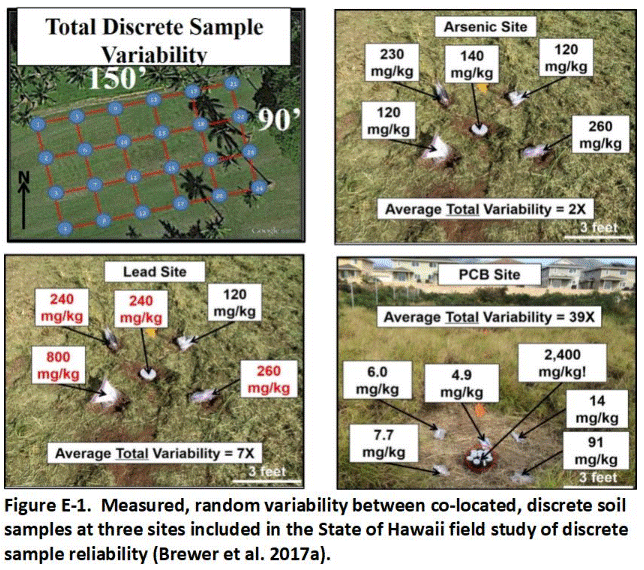 Repeat testing of an exposure area can similarly lead to significantly different estimates of the mean concentration of the contaminant and the associated risk based on a single set of discrete samples (Table E-1; see Brewer et al. 2017a). The mean concentration of arsenic and lead calculated based on independent ten-sample sets of discrete data for the arsenic and lead study sites varied by a factor of approximately two. The mean concentration of PCBs varied by over two orders of magnitude. Brewer et al. (Brewer et al. 2017b) point out that none of the calculated means is reliable, since none of the samples was collected in a manner that ensured representativeness of actual field conditions (refer to Appendix L).
Repeat testing of an exposure area can similarly lead to significantly different estimates of the mean concentration of the contaminant and the associated risk based on a single set of discrete samples (Table E-1; see Brewer et al. 2017a). The mean concentration of arsenic and lead calculated based on independent ten-sample sets of discrete data for the arsenic and lead study sites varied by a factor of approximately two. The mean concentration of PCBs varied by over two orders of magnitude. Brewer et al. (Brewer et al. 2017b) point out that none of the calculated means is reliable, since none of the samples was collected in a manner that ensured representativeness of actual field conditions (refer to Appendix L).
Table E-1. Variability of estimated mean based on repeat testing of ten discrete samples collected from the same area (from Brewer et al. 2017a).
| Study Site | Range Mean (mg/kg) | Range 95% UCL (mg/kg) |
| Site A (arsenic) | 218 to 395 | 278 to 535 |
| Site B (lead) | 170 to 356 | 215 to 469 |
| Site C (PCBs) | 5 to 1,025 | 9.4 to >1,000,000 |
Random variability of contaminant concentrations between co-located samples and between replicate subsamples collected at the laboratory was well known but not well understood. The random nature of a mean contaminant concentration calculated from a single set of discrete sample data went largely went recognized, however. This was due to the lack of routine collection of independent replicate sets of discrete samples from a targeted area of concern for comparison. The periodic collection of replicate sample data to test the precision of the overall sampling method is, in contrast, a required aspect of DU-MIS investigation methods (refer to Appendix L).
Return to the Top of the Page
E.3 IMPLICATIONS OF RELIANCE ON DISCRETE SAMPLE DATA
The results of the Hawaii field study help explain the source of confusion and delays in the completion of environmental projects. The field studies clearly demonstrate that data for a sample collected from a single point are not reliability representative of the surrounding area as a whole. Sample preparation methods at the laboratory are similarly unlikely to capture and represent distributional heterogeneity within the sample itself. As a result, laboratory data are not reliably representative of the sample submitted and the sample submitted is not reliably representative of the area where it was collected. Error in decision making regarding risk or the need for remediation is therefore unknown.
Warnings about the unreliability of discrete sample data are included in numerous USEPA documents and other publications in the 1990s and early 2000s. The USEPA guidance document Preparation of Soil Sampling Protocols (USEPA 1992c) specifically recommends the use of “sampling units,” now referred to as “Decision Units,” and consideration of Gy’s Theory of Sampling for site characterization:
- “Gy’s theory makes use of the concept of sample correctness which is a primary structural property… A sample is correct when all particles in a randomly chosen sampling unit have the same probability of being selected for inclusion in the sample…”
The authors caution reliance on discrete sample data for final decision making (USEPA 1992c):
- “’Grab samples’ or judgmental samples lack the component of correctness; therefore, they are biased. The so-called grab sample is not really a sample but a specimen of the material that might or might not be representative of the sampling unit. Great care must be exercised when interpreting the meaning of these samples.”
E.3.1 Site Characterization Error
Figure E-2 depicts hypothetical isoconcentration patterns that might be generated by a geostatistical mapping program. In this example, the color black is intended to represent areas and volumes of soil that exceed the risk-based screening level for direct exposure. The accuracy of such maps and cross sections is reliant on two critical assumptions regarding the nature of contaminants in the soil: 1) Data for individual points are representative of the immediately surrounding soil and 2) The concentration trend between individual points is linear. Neither of these requirements have been demonstrated to be reliably true for either contaminated soil or sediment, calling into question the reliability of the maps for assessment of risk or development of remedial actions.
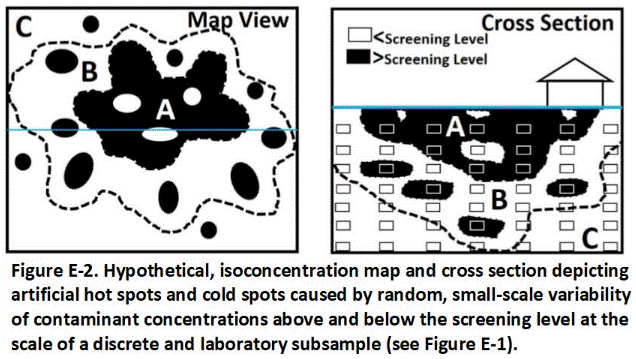
As demonstrated by Brewer et al. (Brewer et al. 2017b), testing of a co-located sample from the same location within a depicted “hot” or “cold” spot or even an alternative subsample from the same sample could yield an entirely different concentration and map pattern. The larger-scale pattern associated with Zone A in Figure E-2 is assumed to be somewhat reliable but includes artificial cold spots due to discrete sample data points that fell below the target screening level. Zone B is characterized by artificial, isolated and random hot spots centered on discrete sample points that by random chance fell above the screening level rather than more accurately depicting the entire zone as contaminated. Zone C is again characterized by a small number of false, isolated hot spots.
Removal of any of the false hot spots in Zones B and C would be unlikely to cause the mean contaminant concentration for each area to be significantly reduced. Failure to remove the false cold spots in Zone A could result in a significantly higher post-remediation mean contaminant concentration for that are that otherwise predicted by the discrete sample data.
While large-scale patterns generated by a mapping program based on large clusters of points can be reasonably accurate, such maps can present a very false sense of small-scale data resolution and contaminant concentration predictability within the mapped area. This can lead to significant errors in the assessment of risk and the development of remedial action plans.
The potential for such errors was pointed out in multiple, early USEPA guidance documents but largely ignored or misunderstood (see Brewer et al. 2017b). As discussed in Appendix D, Multi Increment (MI) sampling methods, which form the basis of this guidance document, were specifically developed to overcome these inherent shortcomings of discrete sampling methods and provide more reliable and defensible data for environmental investigations.
E.3.2 Risk Assessment Error
Risk is assessed based on the mean or “true” concentration of the contaminant for the targeted DU area and volume of soil or other particulate matter as a whole (USEPA 1987, 1988c, 1989b, c, e, f, 1991g, 1992c, 2014f; refer to Section 3.6 and Appendices B and C). Reliance on a single set of discrete sample data to estimate a mean and assess risk is hampered by multiple limitations (Brewer et al. 2017b): 1) Error in assessment of the lateral and vertical extent of contamination (see Section E.3.1); 2) Inadequate number of points represented to reliably capture distributional heterogeneity; 3) Inadequate mass of soil collected in field; 4) Inadequate processing of samples in laboratory; and 5) Lack of replicate sets of discrete sample data to assess precision of calculated mean and the overall sampling method.
A 95% UCL of the mean is often used by risk assessors to compensate for the oftentimes highly variable discrete sample data provided to them. As discussed in Appendix D, decades of experience in the mining industry and detailed field studies have clearly demonstrated this approach to be unreliable. This is because the 95% UCL only addresses potential error in the statistical test employed to estimate a mean for the data set provided. Error in the data set itself due to poor sample collection methods in the field can only be evaluated through the collection of replicate sets of discrete sample data for comparison.
This is not traditionally done for discrete samples due to the cost involved and erroneous assumptions regarding the application of classical statistical methods to particulate matter. Error in assessment of risk based on reliance on a single set of discrete sample data is therefore unknown. Comparison of 95% UCLs calculated for replicate sets of discrete sample data collected from the same DU had demonstrated that this error can indeed be very high (see Brewer et al. 2017a, b).
Collection of a single, MI sample in accordance with Gy’s Theory of Sampling to address compositional and distributional heterogeneity replaces and significantly improves on the use of a 95% UCL calculated for a single set of discrete sample data. Testing of field sampling error through the collection of replicate samples is, in contrast to discrete sampling methods, an integral part of MI sampling methods. While possible, calculation and use of a 95% UCL for replicate MI sample data is discouraged except under limited circumstances and by risk assessors trained with training in Gy’s Theory of Sampling (refer to Appendix L).Return to the Top of the Page
E.4 CONTAMINATION ZONES
The three distinct zones of contamination depicted in Figure E-2 are characteristic of many contaminated sites. Large-scale contaminant concentrations patterns generated by computer mapping programs between contaminant zones can be real. Small-scale patterns within individual zones are often artificial and not representative of actual conditions in the field. Recognizing these zones at sites where discrete sample data are available and understanding the limitations of the data can aid in the design of a more detailed DU-MIS investigation as well as assist in initial remediation actions.
Figure E-3 depicts the random, small-scale variability at the scale of a discrete sample within each of the three zones of heterogeneity common to most contaminated sites. Zone A represents the source area, where the main mass of contamination was released or buried. The mean concentration of the contaminant within this zone as a whole is well above the target screening level, indicating that remediation is required. The concentration of the contaminant at the majority of potential discrete sample points within this zone also exceeds the target screening level (black squares in Figure E-3). It is possible, however, that the concentration at some discrete points within the mass of soil will be below the screening level (white squares in Figure E-3). These points represent false or artificial “cold spots.” The volume of such spots spots is greatly exaggerated by isoconcentration mapping contours (see Figure E-2; Brewer et al. 2017a,b).
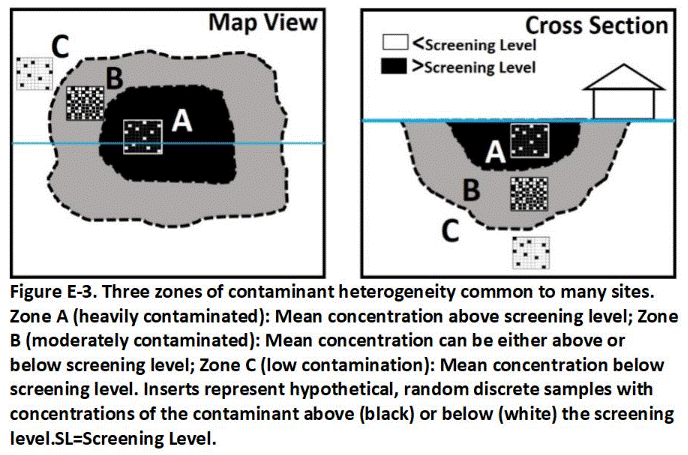
Zone C represents the clean periphery area, where the mean concentration of the contaminant is below the target screening level. Within this zone, the concentration of the contaminant in soil at majority of discrete points will be well below the target screening level. It is possible, however that the concentration of the contaminant at some discrete points will be above the screening level (black spots in figure). Such “hot spots” are again artificial and reflect the heterogeneous nature of contaminant distribution within this area. The volume of such spots is similarly exaggerated by isoconcentration mapping contours and can lead to erroneous decisions for surgical removal of hot spots, even though no remedial action is required for this area (see Figure E-2).
The mean concentration of the contaminant within Zone B of Figure E-3 is higher than Zone C but lower than in Zone A and might or might not exceed the target screening level. This zone is characterized by a mix of discrete sample data both above and below the target screening level. Data for co-located samples collected around individual points can fall both below and above the target screening levels. Isoconcentration mapping programs are unable to account for this small-scale, random variability. As a result, the programs again generate a false pattern of large, seemingly isolated “hot spots” and “cold spots” with the area (see Figure E-2). If an independent set of co-located, discrete samples was collected from the same area then a similar pattern of isolated “hot spots” and “cold spots” would appear, but they would be in different places.
This characteristic makes Zone B-type contamination especially difficult to characterize using discrete sample data. A failure to recognize false “hot spots” on maps of discrete sample data can lead to erroneous attempts surgically remove small areas of presumed contaminated soil to reduce the overall mean concentration of the contaminant for the targeted area. Confirmation samples collected around such excavations often surprisingly exceed the screening level, resulting in decisions to continue excavation with no clear end point in site. Even after removal of all apparent hot spots, retesting of the area using more reliable and risk-based DU-MIS sampling methods will often indicate that removal of the soil did not result in a substantial reduction in the mean contaminant concentration or in the associated risk (refer to Brewer et al. 2017b).Return to the Top of the Page
E.5 USE OF DISCRETE SAMPLE DATA FOR PRELIMINARY SCREENING
Although discrete sample data are not reliable for final decision, existing sample data can sometimes be useful for preliminary identification of large areas of contamination and designation of DUs for the collection of more reliable DU-MIS data. This can aid in initial removal of heavy contamination or in the designation of DUs to help isolate areas of heavy contamination for optimization of remediation. The boundaries depicted between areas of heavy, moderate and low-level contamination should be considered preliminary, however, and verified with more reliable DU-MIS confirmation data. As discussed in Appendix E, seemingly isolated “hot spots” and “cold spots” based on a single or otherwise small subset of discrete sample data are likely to be false artifacts of the mapping programs inability to consider random, small-scale heterogeneity and should generally be ignored.
Note that this is true for any type of isoconcentration map based on discrete, environmental sample data, including maps for subsurface soil vapor and groundwater data. Specific errors often encountered in unadjusted, isocontour maps include:
- Artificial “hot spots” and “cold spots” caused by random, small-scale variability of contaminant concentrations at the scale of a discrete sample;
- Erroneous “zero” isocontours around the perimeter of contaminated areas due a lack of outward data points;
- Inherent lack of precision of isocontour placement;
- False conclusions regarding risk; and
- False conclusions regarding the need for or adequate completion of remediation.
Unrecognized, these errors can lead to a false sense of precision in computer-generated isocontour maps and lead to erroneous decisions regarding the need to continue or halt site investigations or remedial actions. This includes calls for remediation of isolated “hot spots” based on single or small numbers of discrete samples and premature termination of site investigations or remedial actions due to false “cold spots” in the discrete sample data.
Isocontour maps should be adjusted to reflect site knowledge and professional judgment not reflected in computer-generated maps. Such adjustments are not possible in existing computer programs and must be done by hand. Boundaries between apparent large-scale patterns should necessarily be dashed. Small-scale heterogeneity within larger-scale patterns generated by small numbers of discrete sample points should not be presented on final maps included in the report.
For example, Figure E-4 depicts a nine-acre (four-hectare) site formerly used for storing and mixing pesticides. The northern area of the site was known to be heavily contaminated with arsenic based on previous collection of both discrete and MI samples. The exact area of elevated arsenic was uncertain based on previous testing although the area of the former mixing shed was most suspect. No obvious signs of contamination were recognizable in the field.
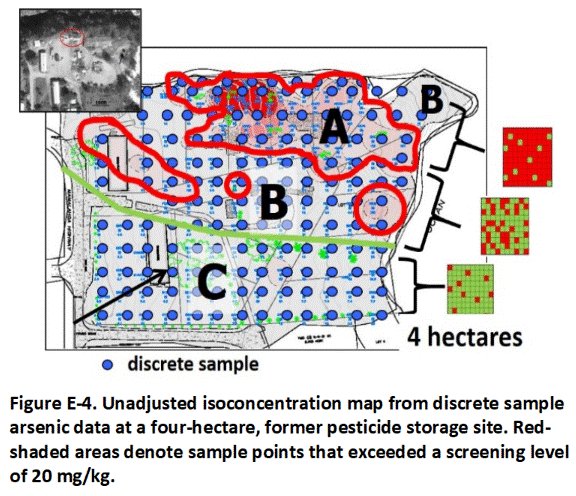
Approximately 90, large-mass, discrete surface soil samples (0 to 15 cm or 0 to 6 in) were collected from a 15 m (16 yd) grid across the site. Each discrete sample was collected from multiple points around each grid point in order to help address random, small-scale heterogeneity and increase data representativeness. Samples were analyzed using a portable XRF. A subset of samples was analyzed in a laboratory for comparison.
As can be seen in the figure, the XRF helped identify at least one large source area of arsenic-contaminated soil in the northern part of the site. Smaller clusters of discrete samples with higher reported levels of arsenic might or might not be reflective of actual conditions in the field. False patterns of higher and lower levels of contamination can be produced by samples that are too small to capture and smooth out random heterogeneity of contaminant distribution in soil (refer to Appendix E).
Three distinct areas of arsenic contamination are apparent (Figure E-5):
- A) Concentration of arsenic in the majority of discrete samples below screening level 20 mg/kg, with occasional “outliers” that exceed this value;
- B) Concentration of arsenic randomly above and below 20 mg/kg; and
- C) Concentration of arsenic above 20 mg/kg in the majority of discrete samples with random “outliers” below this value.
The most heavily contaminated soil in Area C corresponds to location of a former pesticide mixing area at the facility (denoted by red circle on the inserted 1979 aerial photo). Smaller-scale patterns of apparent hot spots within Area B are interpreted to be artifacts of random, small-scale heterogeneity and might or might not be reproducible.
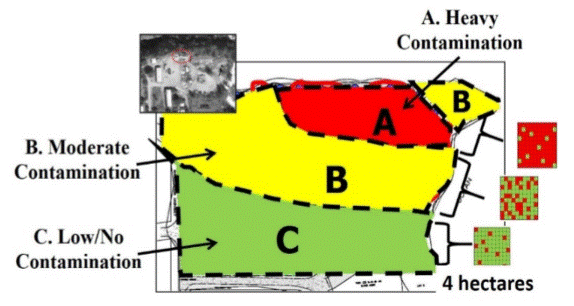
Figure E-5. Adjusted Arsenic Isoconcentration Map for a Former Pesticide Storage Site. The adjusted map more accurately reflects the resolution of arsenic distribution in soil across the site that can be reliably extracted from the discrete sample data.
These types of discrete sample data maps can be used to help designate DUs and carry out a more reliable and higher resolution characterization of the site. Figure E-6 depicts Exposure Area DUs designated in Zone C of the site based on the maximum-allowed exposure area agreed to by the risk assessor (e.g., one acre). Smaller DUs are designated in the heavily contaminated Zone A area. A mix of DU sizes are placed in the Zone B area, based on the judgement of the field team. As an alternative, removal of the soil in the Zone A can occur prior to additional sample collection and larger exposure area-size DUs designated in the area for confirmation of cleanup.
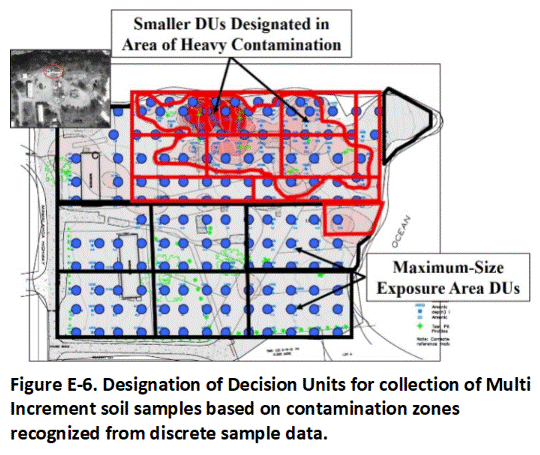
Preliminary maps could also be used to carry out initial remediation actions, for example removal of soil from the heavily contaminated area, followed up with a DU-MIS investigation to confirm removal and assess the need for additional actions. The cost-benefit of basing initial remedial actions on discrete sample data requires significant experience and coordination between both the risk assessor and the remediation specialist.
APPENDIX F. COLLECTION OF SURFACE SOIL SAMPLESReturn to the Top of the Page
F.1 LOCATING DU INCREMENT COLLECTION POINTS
Use a Global Positioning System (GPS) devise to record the corners of the Decision Units (DUs) or, if irregular, enough points to delineate the DU shape. Use the GPS data and field measurements to prepare a to-scale map of all DU shapes and locations. Include a bar scale and compass point. Note that GPS location information can be several feet off. Use of tape measures or equivalent approaches in the field is recommended to document the exact dimensions of a DU. If there are buildings or other fixed structures on the site near the DUs, use physical measurements from the structures to assist in documenting the location of the DUs in the field. Satellite or aerial images that depict the DUs are also very useful and should be included if easily available.
Increment collection points are determined by hypothetically dividing the DU in small, square cells equal in number to the target number of increments (Figure F-1). A single increment is collected from the center point of each cell.
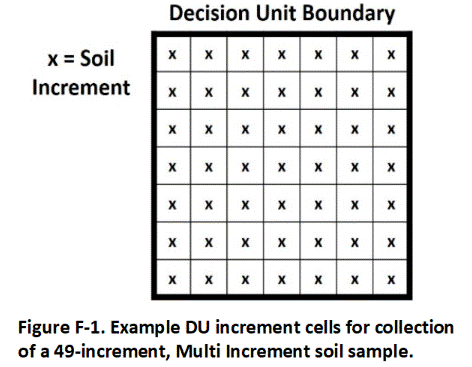
The dimensions of each increment collection cell are calculated using the following equation (see Section 3.6.2):
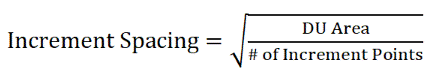
This simulates division of the DU into individual increment collection cells, with a single increment collected from the center of each cell. After calculating the square root of this area, the length of each side of the cell is obtained (assuming square cells). Individual increment collection points are spaced at a distance equal to the side of each collection cell. The same spacing applies to subsurface increments if the thickness of the DU exceeds this value.
Documenting or flagging the location of every individual increment collected within a DU is not necessary, although spacing and number of increments collected per DU should be stated in the site investigation report. Flagging the locations of increment rows along two parallel sides of a DU and progressively moving a tape measure marked with increment collection locations (or careful pacing) between opposing flags across the DU as increments are collected can significantly expedite field work. A few rows of flags can also be placed within large or long DUs as needed to help guide sample collection.
Use of a GPS in the absence of flags can expedite the location and collection of increments for very large DUs (e.g., tens or hundreds of acres), where error in increment location within a few feet is acceptable and where pacing might not be accurate or practical due to vegetation, topography, or other access issue.
Increments should be collected in an evenly spaced zig-zag pattern in long narrow DUs, as depicted in Figure F-2. A tape measure or rope with flags tied at the appropriate spacing can be placed in the DU to assist in increment collection, without the need to flag individual points. Ensure equal increment spacing in all directions.
F.2 INCREMENT AND BULK SAMPLE COLLECTION
Collect a single increment from a random point within the first cell, for example the center. Collect a single increment from the same point in the remaining cells. (Figure F-3). Combine all increments into a single sample and place in an appropriate sample container, most commonly a heavy-duty freezer bag. Alternative methods for the collection of independent, replicate samples from the same DU are discussed in Appendix L.
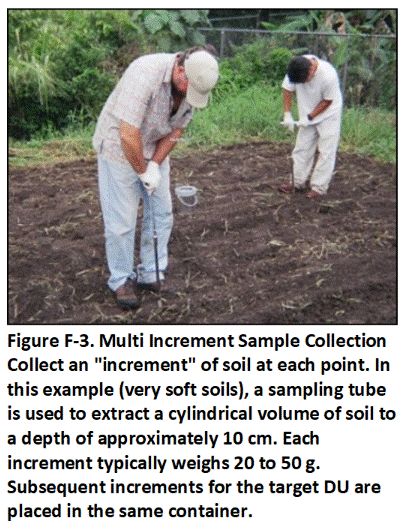
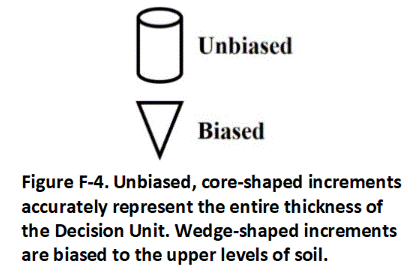
Collect core-shaped increments to ensure unbiased, equal representation of the entire thickness of the subject DU. (Figure F-4). Core-shaped increments can be collected using a soil coring sampler, soil sampling tubes (both preferred), or drills with specialized bits. This ensures equal coverage at all depths of the targeted DU layer. Hand trowels tend to produce wedge-shaped increments, with a bias towards the upper section of the targeted soil and are generally not recommended. If used, an effort should be made to extract core-shaped increments. Using the wrong tools or collecting a sample that contains more soil particles from the top of the targeted DU than the bottom will lead to biased sample results and potentially non-representative data, due to a heterogeneous vertical distribution of contaminants in the soil.

Determine the appropriate increment mass by dividing the target, bulk sample mass by the number of increments to be included in the sample. For example, fifty, 40-gram increments will produce a bulk soil sample with a mass of 2 kg, within the default target mass range of 1 to 3 kg (Figure F-5). Avoid the need to collect a smaller subsample from an excessively large primary sample in the field beyond removal of sticks and large rocks, as this will introduce additional and unknowable error to the resulting laboratory data. Increments for samples to be tested for VOCs should be placed in methanol or another preservation method employed. Refer to Appendix I for additional details.
Do not attempt to collect co-located increments or “splits” of initially collected increments for later, individual testing. The random, small- scale variability of contaminant concentrations within small masses of soil negates the reliability of any given increment to be representative of the immediately surrounding soil (refer to Appendix D). Testing of smaller groupings of increments collected within a single DU (e.g., four groupings of ten increments each) is likewise invalid, since the resulting data cannot be assumed to be representative of the area from which the increments were collected (i.e., number of increments and bulk sample mass too small). Doing so is wasteful of both field time and analytical budgets.
If a greater resolution of contaminant distribution might be required for a targeted area then the initial designated DU should be subdivided into smaller DUs from the start, with a Multi Increment (MI) sample collected from each area (see Appendix C; refer also to Section 3.3). This will avoid delays and mistakes over data interpretation in the future should not add significantly to the time required to collect samples or the overall cost of the project.
The same holds true in cases where significant contamination is identified in a large DU where contamination was not initially anticipated. If a greater resolution is subsequently desired to optimize remedial actions, then the DU should be subdivided accordingly and a proper MI sample collected from each new DU.
Return to the Top of the Page
F.3 SAMPLE COLLECTION TOOLS AND FIELD METHODS
The collection of samples reflects the culmination of significant research and planning prior to initial field activities. It is important that the samples be as technically defensible and representative of site conditions as possible. The tool(s) selected for sample collection must ensure that soil increments are core-shaped or otherwise not biased with respect to depth and of relatively equal mass, and that mass of individual increments be adequate to collectively meet the target bulk mass for the resulting samples.
The collection of samples from exposed surface soil should be relatively straight forward with proper planning and tools. The top 10 to 15 cm (4-6 in) of soil is typically designated as a DU for sample collection and characterization of surface spills as well as assessment of direct exposure risk (USEPA 2011d; CAEPA, 2013). The same tool used for surface soils can often be used to collect deeper, near-surface soil samples (e.g., 15 to 25 cm (6-10 in)) if included in the site investigation objectives or necessary for vertical delineation of contamination. The collection of samples from DUs layers greater than three feet below the ground surface typically requires the use of drills or other equipment (see Appendix G). Although not typically carried out for surface soils, the field use of methanol or alternative approaches for the collection of MI sample to be tested for VOCs are described in Appendix I.
Soil type, compaction, abundance of rocks, and increment depth typically drive selection of the most appropriate tool for a given site. A simple sampling tube is generally most appropriate for relatively non-compacted, fine-grained soils. Sampling tubes with core catchers or using a trowel might be most appropriate for very loose, sandy soil, although care must be taken with the latter to collect increments that are not biased with depth. An electric drill with an auger bit specifically designed to remove cuttings can allow the rapid collection of increments and samples in fine-grained, semi-compact soils. A sample tube with a slide hammer, a mattock, electric hammer, or in some cases even a backhoe might be required to collect samples in very compact or very gravelly soil.
The following, more detailed discussion of sampling collection methods is presented in terms of tool options for various soil types and field conditions. The examples provided are based in part on the field experience of the authors of this guidance. An inspection of the site prior to sample collection to assess soil conditions is imperative. Multiple types of tools should be taken when actual field work commences to address unanticipated field conditions and avoid delays, as well as ensure that representative samples can be collected.
Wire flags, marked tape measures or rope and rolling measures can be used to mark individual increment collection locations and expedite sample collection. Ensure that all sampling devices are of sufficient quality to avoid contamination of the samples being collected with paint, chrome plating, grease or other material. Sampling equipment should be either easy to decontaminate or cost-effective enough to be disposable.
F.3.1 Soft, Fine- to Coarse-Grained Soils
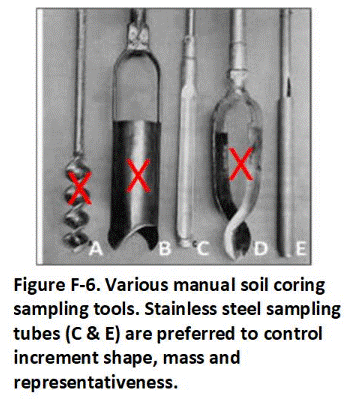 Manual sampling tubes that extract core-shaped increments are preferred for the collection of surface samples (“C” and “E” in Figure F-6) and screw-type drills (“A” in Figure F-6). Stainless steel soil coring devices rather than augers are recommended for the collection of MI samples. Screw-type drills (“A” in Figure F-6) and augers tools (“B” and “D” in Figure F-6) are less reliable for removal of core-shaped increments of consistent mass and should be avoided when possible.
Manual sampling tubes that extract core-shaped increments are preferred for the collection of surface samples (“C” and “E” in Figure F-6) and screw-type drills (“A” in Figure F-6). Stainless steel soil coring devices rather than augers are recommended for the collection of MI samples. Screw-type drills (“A” in Figure F-6) and augers tools (“B” and “D” in Figure F-6) are less reliable for removal of core-shaped increments of consistent mass and should be avoided when possible.
Small-diameter (e.g., 2 cm (0.8 in)) sampling tubes are generally preferable in soft or loose, clayey to sandy soils that are not rocky (Figure F-7). The tubes are light weight, durable, simple to use and wash and minimize visual disturbance of a site after samples are collected. The tools are also very useful for the collection of samples from very large DUs, where considerable walking is required, and for cases when only one person is collecting samples. They also serve as a useful backup or alternative to a drill (see below) should battery, fuel or other equipment problems arise in the field. Importantly, 2-cm (0.8 in) diameter tubes also allow for the collection of 50- to 75-gram increments from the upper 10 to 15 cm (4-6 in) of soil, ideal for the collection of a 50-increment, 1 to 3 kg sample.
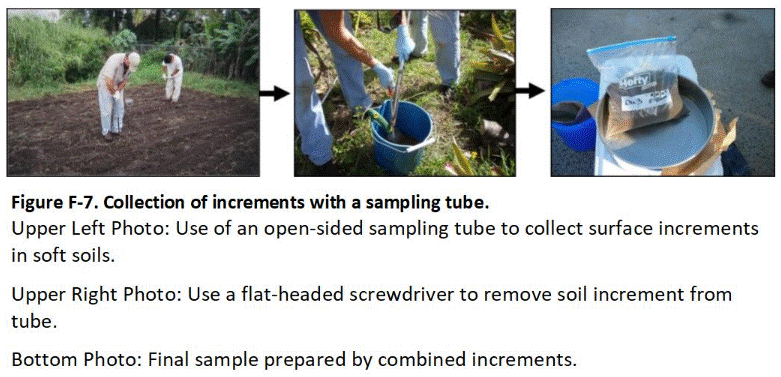
Larger diameter tools (e.g., 5 cm (2 in) and larger) collect a proportionately larger amount of soil from a single location, resulting in a sample that could exceed the 3 kg recommended limit. Check with the laboratory to know the maximum total mass of bulk sample they are willing to accept and process using their standard MI sample processing protocols. Higher processing fees might be assessed for overly large samples. Select a core diameter that, in conjunction with the targeted DU depth/thickness, will result in a bulk sample mass above the recommended 1 kg minimum and below the laboratory manageable maximum. Note that larger mass bulk samples are generally more representative, so the choice of the sample core diameter is a balance between what is effective to utilize in the field, the amount of contaminant heterogeneity expected and cost for the laboratory processing.
Sampling tubes are utilized with extension rods and T-handle attachments (see Figure F-7).The tube is twisted into the ground to the desired depth, cutting into and retaining the soil in the hollow, open-face core barrel. The tube is then withdrawn to extract the increment from the ground. The increment is then removed and placed into a collection bucket for the DU sample. A flat-edged screwdriver or similar tool is useful for removing an increment of clayey or hard-packed soil.
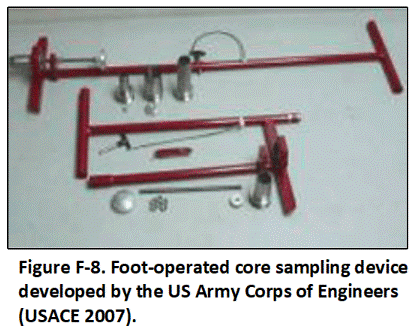 A foot-assisted coring tool can also be useful for the collection of increments in soft but cohesive fine-grained soils (Figure F-8). The core barrel is pushed into the soil and retracted. The increment is extruded into the container with a spring-operated plunger. These tools can allow the very rapid collection of MI samples in uncompacted soils without gravel.
A foot-assisted coring tool can also be useful for the collection of increments in soft but cohesive fine-grained soils (Figure F-8). The core barrel is pushed into the soil and retracted. The increment is extruded into the container with a spring-operated plunger. These tools can allow the very rapid collection of MI samples in uncompacted soils without gravel.
Sampling tubes and core barrels such as those shown above do not work well in dry, loose soils that lack sufficient cohesion and will not allow particles to be retained or removed by the tool. Use of core catchers, if available for the coring device in use, might be an effective alternative. Alternatively, scoops with flat bottoms or similar hand tools are generally utilized in these conditions (Figure F-9). If scoops or trowels are utilized, it is important to remember that the goal is to remove similar-sized core-shaped increments in the DU (increments of uniform diameter through the vertical depth targeted), as well as limit increment mass to that needed to prepare a bulk 1 to 3 kg MI sample. The flat lip of the scoops shown in Figure F-9 can help ensure that wedge-shaped increments are not collected (see Figure F-5).
F.3.2 Moderately Compact, Fine- to Coarse-Grained Soils
A cordless drill used in conjunction with a paper plate can be time and cost-effective for semi-compact to hard-packed soils without significant gravel, but can require two people unless a specially designed foot plate is used. Collection of a 50-increment sample can take as little as 10 to 15 minutes in open areas.
 Figure F-10 depicts the use of a heavy-duty, battery-powered drill (e.g., Milwaukee or Grainger models) with a 28 volt battery and a 2.5 cm (1 in) drill bit to collect sample increments. Cheaper and less durable drills intended for home use are prone to overheat or quickly drain batteries, especially in clayey or hard-packed soils. Commercial-grade drills can generally be used for up to 100 increments per battery charge. Field chargers are available for vehicles.
Figure F-10 depicts the use of a heavy-duty, battery-powered drill (e.g., Milwaukee or Grainger models) with a 28 volt battery and a 2.5 cm (1 in) drill bit to collect sample increments. Cheaper and less durable drills intended for home use are prone to overheat or quickly drain batteries, especially in clayey or hard-packed soils. Commercial-grade drills can generally be used for up to 100 increments per battery charge. Field chargers are available for vehicles.
To collect an increment, place a paper plate with a pre-cut 2.5 cm (1 in) hole on top of the target point. The center of the plate must be held down to keep soil from piling up under the plate. Keep any tears in the paper around the hole pressed together to minimize soil loss. The use of pre-drilled, metallic or plastic plates is also possible provided that the plates do not contain contaminants of potential concern for the targeted DU. Soil sampling buckets that include a hole drilled in the base are also available from some vendors.
Keep the drill vertical and advance the bit to the target depth (e.g., marked with tape on bit) as soil piles up on the plate. Hold the drill firmly so that the drill doesn’t lurch and strike the second person if gravel or hard-packed soil is encountered. Wear nitrile or latex gloves and change gloves between DUs (not shown in demonstration photos). Progress the drill to the targeted depth. Empty the soil into a sample container dedicated to the DU sample being collected (e.g., decontaminated plastic bucket) and move to the next increment collection point. The drill bit does not need to be decontaminated between increments to be combined into a single sample but must be decontaminated between replicate samples and between DUs.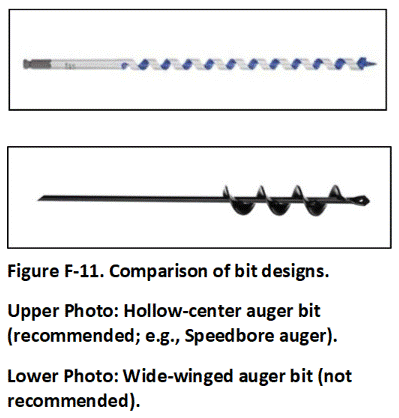
Use a hollow auger bit (e.g., Speedbore bit) to improve soil removal from the ground and control the collected soil mass (see Figure F-11). These bits generally produce 30 to 50 grams of soil per 15 cm (6 in) depth. Hollow center auger bits typically work better in the field than wide-flight bits (Figure F-11). Hollow auger bits are designed to more efficiently remove cuttings from a boring without bringing up excess soil. The area where soil is removed from a boring is less easy to control with a wide-flight bit, and the bits can either bring up too much or too little soil with respect to the target increment mass.
Heavy-duty paper plates work well in the field under dry conditions (see Figure F-10). Pre-cut holes save field time; several plates might be required per DU if the plate tears excessively during increment collection. Wooden or metal plates might also be useful. Care should be taken not to get fragments of the plate into the sample due to potential interference in laboratory analysis from glue, plastic or metal. Sampling kits for drills are also available from soil testing supply stores. The kits include a metal foot plate with a drill guide that attaches to the base of a sampling bucket, with increments directly deposited into the bucket (e.g., AMS Compacted Soil Sampler).
Heavier duty drills with portable generators are also an option (Figure F-12). This setup avoids the need for recharging batteries and can drill through more compact soils. These drills should only be used by an experienced person. The sudden torque of the drill if a rock or compact object is encountered can cause severe injury to the wrists. The use of an electric hammer drill with a spade bit is instead recommended (refer to Section F.3.3). Caution should also be taken to avoid bit sizes and designs that result in the collection of excessively large increments and bulk samples.
A manual, hydraulic, or electric slide hammer can also be used to advance the coring device into shallow soil (Figure F-13). Slide hammers are effective for collecting harder packed soils but require considerable effort and energy to use in the field. A weighted slide hammer is physically lifted and lowered along a guide rod to drive the attached tool string into the ground to collect shallow soil samples.

F.3.3 Very Compact or Gravelly Soil
A hammer-action electric drill with a spade bit is recommended for the collection of samples from very hard or gravelly soils (Figure F-14). Generators, drills and bits are usually available for rent from a local hardware store. Activate the drill and use the hammer action to insert the spade in the ground and to push the soil to one side as the bit moves toward the targeted depth, opening up a small gap in the ground (Figure F-15). Use a trowel to remove a core- or slab-shaped increment from the side of the opening, being careful to collect an equal amount of soil from all depths. Remove large rocks from the bulk MI sample as it is being collected. Ensure that an adequate amount of the target soil particle size (e.g., <2 mm) is collected per increment to prepare a bulk sample. Collect a similar mass of soil at each increment collection point adequate to prepare a 1 to 3 kg bulk sample.

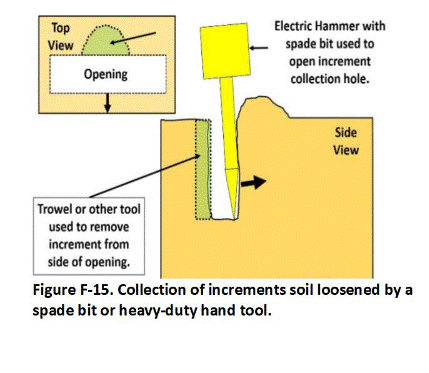
 Other options include the use of a mattock or heavy-duty rock hammer to loosen a core-shaped volume of soil from the hard-packed ground (Figure F-16). A trowel is then used to collect the increment. This avoids the need to carry and rely on an electric drill and generator but adds significantly to the time and effort required to collect samples.
Other options include the use of a mattock or heavy-duty rock hammer to loosen a core-shaped volume of soil from the hard-packed ground (Figure F-16). A trowel is then used to collect the increment. This avoids the need to carry and rely on an electric drill and generator but adds significantly to the time and effort required to collect samples.
Heavier duty hand tools can also be useful to break through hard surfaces or cut through concrete or asphalt to access underlying soil. This can be very labor intensive and can significantly slow down sample collection activities. Chisel or spade bits used with an electric hammer (see Figure F-14) or a tunnel bit used with an electric drill (Figure F-17) can be used to remove plugs of asphalt or concrete more rapidly. Ideally, this should be done ahead of time to expedite actual sample collection.
Tunnel bits are not recommended for the collection of increments from hard-packed or gravelly soil, despite the ability to extract a core-shaped plug of material. The mass of material collected inside a standard 7 cm (3 in) bit exceeds that needed for the collection of a 1 to 3 kg sample, requiring subsampling of the bulk sample in the field or laboratory to reduce mass for additional processing. Removing soil and large gravel from the bit can also be tedious and time consuming.
APPENDIX G. COLLECTION OF SUBSURFACE SOIL SAMPLES
Designation of Decision Units (DUs) for the collection of subsurface Multi Increment (MI) samples is discussed in Section C-7 of Appendix C. “Subsurface” soil is generally considered to be soil that is below 25 to 100 cm (10-39 in) depth or soil that is otherwise difficult to access with standard tools used for the collection of surface samples. A subsurface DU can be thought of as a surface DU that is covered with an additional layer of soil. The fact that the targeted DU soil layer is covered by additional soil does not negate the need to collect a high-quality sample. The same applies to characterization of sediment that is covered by a layer of water.
Shallow subsurface soil (e.g., <25-50 cm (<10-20 in)) might be accessible using a sampling tube, slide hammer, or electric drills as described in Appendix F for surface soils. Hand tools such as shovels could also be used to access deeper soil. The collection of increments and samples below this depth or from hard-packed soils will generally require the use of a push rig able to collect continuous cores. A backhoe or similar equipment can also be used for trenching or pot holing to gain access to deeper soil.
Overviews of push rigs and other drilling equipment are provided in ASTM Standard D 6169 (ASTM 2005b) and ASTM Standard D 6286 (ASTM 2006, b, c). Direct push technologies can be used to collect samples to depths of up to 10 m (11 yd) below ground surface or more, depending on the compaction of the soil and the presence of rocks. Auger drilling can reach depths of 30 m (33 yd) or more. Rotary drilling can reach depths of 300 m (328 yd) or more.
Each of these technologies is discussed in more detail below. Direct-push methods are generally necessary for the collection of subsurface MI samples. Although included in the discussions below, auger and rotary drilling is more amenable to geotechnical investigations or the installation of monitoring wells. This is due to the difficulty in collecting continuous cores of a manageable size, as well as the expense, effort, and space required to operate the equipment. Drill cuttings and cores from such equipment might, however, be useful for initial screening of subsurface conditions and the need for a more intensive investigation (e.g., presence of absence of staining, approximate boundaries between contaminated fill and native soil, identification of contaminants of potential concern, etc.).
Return to the Top of the Page
G.1 EXPLORATORY PITS, TRENCHES AND BORINGS
The installation of large numbers of boreholes and collection of Multi Increment samples from large, subsurface volumes of soil might not be possible at operating properties with operating buildings and other limitations to access. Exploratory pits, trenches and borings can be very use for initial investigation of subsurface contamination (Figure G-1). Look for changes in soil type, the presence of debris, staining, odors and other indications of possible contamination. Collect samples from suspect layers to identify contaminants of potential concern and identify potential clean boundaries. Consider use of a portable XRF, Photo Ionization Detector, immunoassay kits and other field screening tools to assist in the initial delineation of contaminated areas. While not normally reliable to complete a full risk-based investigation of site conditions, the observations made and data collected can be used to approximate the lateral and vertical extent of heavy contamination and designated DUs for a more thorough investigation.
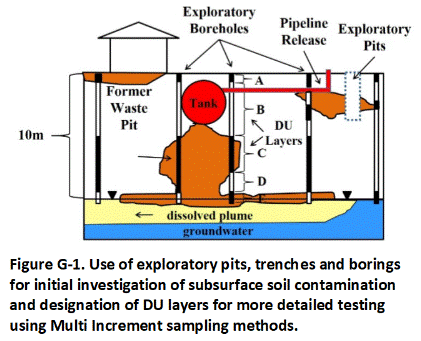 Continuous cores should be collected from boreholes using a direct-push rig whenever possible. Divide the core into targeted DU layers for testing. Submit the entire section of the core for a DU layer to the laboratory for processing and tested as a single sample if possible. “Discrete” samples from single points within the core should not be collected due to the unreliability of the resulting data to represent the targeted DU layer (see Appendix E).
Continuous cores should be collected from boreholes using a direct-push rig whenever possible. Divide the core into targeted DU layers for testing. Submit the entire section of the core for a DU layer to the laboratory for processing and tested as a single sample if possible. “Discrete” samples from single points within the core should not be collected due to the unreliability of the resulting data to represent the targeted DU layer (see Appendix E).
The collection of a representative subsample from a core might be required if the total mass of the core interval exceeds the recommended 1 to 3 kg bulk sample mass. Methods for collecting representative subsamples from cores are discussed in Section G-3. Samples to be tested for VOCs should be placed in methanol in the field or otherwise preserved for extraction and testing at the laboratory (refer to Appendix I).
Be aware of the possibility for initial random pits, trenches or borings to miss widespread but discontinuous subsurface contamination. Document the limitations on data reliability for recommendations of additional action. Testing of subsurface soil can also be carried out following excavation by temporarily storing the soil in stockpiles (see Appendix H).Return to the Top of the Page
G.2 DIRECT-PUSH TECHNOLOGIES
Direct push technologies are a category of equipment that push or drive small-diameter hollow steel rods into the subsurface without rotating the drill rods (Figure G-2). Direct push drilling can yield high-quality continuous cores of soil from targeted depth intervals quickly and cost effectively in the right type of soil conditions and is ideal for MI sampling strategies. Push rigs can also be used to collect soil gas or groundwater samples. Smaller track-mounted rigs can be used for sampling areas with limited access. These rigs are also normally remote controlled and can be programmed to collect increments from a pre-established grid.
A hydraulic hammer is used to progressively drive the steel rods into the soil, with the weight of the drill rig used to provide a constant force on the drill string (Figure G-3). Casing is advanced with a solid point held in place by an internal rod. A 4 cm to 6 cm (1.6-2.4 in) diameter inner rod and core barrel is typically combined with an 8 cm to 10 cm (3.2-3.9 in) diameter outer casing. As each section of the rod is advanced into the subsurface, another section of casing and rod can be attached to achieve greater depths. Three- to five-foot drive rods and samplers are typically used, depending on the depth and thickness of the targeted DU layers. Multiple drives might be required to extract the full length of core needed.
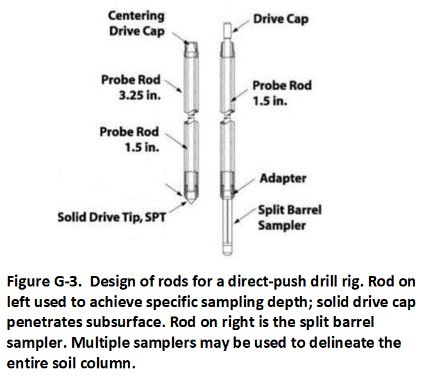
The steel rods and driving tip are pulled from the subsurface when the top of the desired soil interval (i.e., top of DU layer) is reached, with the outer casing left in place. The solid point is removed from the end of the inner rod and a split-spoon or open-barrel sampler is attached.
A split-spoon sampler is a stainless steel, machined, hollow cylinder that can be opened lengthwise into two halves (Figure G-4). Split spoons can be used with hand-operated slide hammers, push rigs or larger drilling rigs. The cylinder is fitted with threaded ends. A cutting shoe is connected to the downhole end and a driving cap is connected to the uphole end. Split-spoon samplers can be lined with a clear Teflon or polyethylene lining to help keep cores intact after the sampler is opened. Stainless steel or brass tubes are also sometimes used, although they are less amenable for the collection of MI samples.
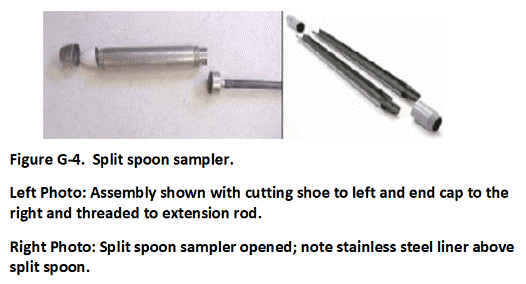
After the coring device is attached, the drill string is placed back into the casing and driven to the desired depth. A hydraulic hammer can be used in conjunction with the push rig for compact soils. The drive rod can be marked to help monitor depth. A drop hammer can be used to measure blow counts as part of a Standard Penetration Test if required as part of the investigation (ASTM, 2011b). For example, a 65 kg hammer is dropped 75 cm (30 in) and blows counted to advance each of three consecutive, 15 cm (6 in) increments for a total of 45 cm (18 in). The resulting data are used to help evaluate structural properties of the soil, including consistency, in-situ strength, and susceptibility to liquefaction.
The drill string is retracted and brought back the surface after the base of the targeted interval has been reached under ideal circumstances. A continuous and relatively undisturbed core is ideally collected within the device. The sample barrel is opened and the core exposed (Figure G-5). The top of the plastic liner, if used, is cut away to allow access to the soil. Estimation of the boundaries between targeted DU layers might be required in cases where a complete core is not extracted, or where compaction during collection distorts the original thickness of the layers.

Return to the Top of the Page
G.3 BOREHOLE CORE INCREMENT SUBSAMPLES
Each core section represents a single increment for a targeted DU layer in the same manner that a smaller core of soil collected from a shallow surface DU represents a single increment for that DU. Preliminary extraction and testing of cores from single, “exploratory” borings can carried out to help designate subsurface DU layers for more detailed testing and identify contaminants of potential concern (refer to Section G-1 and Figure G-1). If done, the entire section of core for a targeted interval should be submitted to the laboratory for processing as a single sample or a representative subsample collected along the entire length of the core section. The collection and individual testing of single, “discrete” subsamples from specific points within a core will not provide reliably representative data and should be avoided (refer to Appendix E).
Targeted DU layers are identified and marked in the core (Figure G-6). The mass of an individual core increment collected with a push rig is typically too large for use in preparation of a bulk MI sample and field subsampling of the core increments is required. Each increment for an individual DU layer is subsampled, with the extracted soil placed in a container specific to that layer. The subsample should be collected in a manner that ensures it is representative of the entire, targeted DU layer. This can be accomplished by cutting a narrow wedge of soil from the entire length of the increment interval or by removing regularly spaced plugs of consistent mass from the increment (e.g., 5 to 10 grams; Figure G-7).
The MI sample should be collected in a manner such that a proportionally greater subsample mass of soil is collected from cores where the DU layer is thicker in comparison to cores where it is thinner. This will allow the final sample to be representative of the DU layer within the targeted area as a whole. Preparation of a representative sample is accomplished by collecting subsamples in a consistent manner from all cores. For example, the collection of a 5g subsample every 5 cm (2 in) in all cores will result in a final sample mass representative of the total length of the DU layer in the cores as a whole.
Under ideal circumstances the wedge method is preferred since the resulting subsample provides 100% vertical coverage of the core increment. Removal of a continuous wedge from a core might not be possible if rocks are present or loss of volatiles might be an issue, however, and subsampling of core increments using the plug approach is most common.
Increment subsamples are combined in the field to prepare a single sample for the DU layer in the same manner as carried out for surface soil sample. Note that the individual soil plugs are subsamples of a single core increment and do not represent individual increments themselves. Individual subsample plugs cannot be counted towards the total number of increments collected from a subsurface DU layer since they were not collected from independent, random locations. The minimum recommended number of increments for testing of a DU (e.g., 30 to 75; see Section 3.6.2) does not apply to subsampling of core increments using subsample plugs. The precision of the subsampling method is instead tested by the collection of replicate (triplicate) sets of subsamples from the same core in 10% of the DUs, as described below.
The mass of soil included in a single core wedge or as the sum of plugs removed from a single core increment is dependent on target sample mass for final DU layer in the same manner as done for a surface sample. For example, if a 1.5 kg bulk sample is desired and 30 core increments are to be collected to represent a DU layer, then the subsample mass for each core increment should total approximately 50 grams. Careful consideration of the soil subsample mass collected from each core increment prior to subsampling is critical to ensure that mass of the resulting bulk sample will be adequate to meet target requirements (e.g., 1 to 3 kg) but not so large that additional subsampling in the field or laboratory will be required.
If a core wedge cannot be collected, then the target subsample mass should be collected from what is anticipated to be a representative number of points within the core increment layer. For example, if the collection of an approximately 1 kg bulk MI sample from a 25 cm (10 in) thick DU layer is targeted, and thirty increment cores are to be collected in the DU, then six, 5-gram plugs at a spacing of two inches could be extracted from each of the 30, 25 cm-foot thick core increments for a total bulk MI sample mass of approximately 900 grams for the DU layer. The mass of soil removed from each individual core increment should be kept constant, assuming a constant DU layer thickness. Maintain consistent wedge width or plug spacing for subsampling of core increments collected from DU layers with varying thicknesses between borings.
The collection of replicate samples to evaluate the precision of both increment subsampling and the overall sampling approach is recommended (see Appendix L). At a minimum, replicate sets of increment subsamples (e.g., triplicates) should be collected from one or more of the targeted DU layers and combined into independent samples for testing. For example, replicate sets of subsamples can be collected from different sites of the same core and reserved for combination into single, independent samples. If the resulting data are reasonably consistent (e.g., RSD <35%) then the precision of the subsampling methods used can be considered to be good (see Appendix L). This allows both the precision of the subsampling method as well as the precision of the overall sampling method to be evaluated. Independent sets of borings are used to collect replicate samples in select DUs to test the precision of the overall approach, in the same manner as done for surface samples.
Return to the Top of the Page
G.4 PIT OR TRENCH SAMPLE COLLECTION
The use of pits or trenches to collect samples might be required in situations where considerable debris, rubble, or rock create obstructions in the subsurface. Pits and trenches can also provide useful information on the nature of subsurface soils within a DU prior to a more detailed investigation.
Pits can also be used to collect sample increments from shallow subsurface DU layers in cases where a push rig is not available or at sites where heavy equipment is already available and more cost- or time-beneficial to use (Figure G-8). Denote the tops and bottoms of targeted DU layers using a measuring tape or stick. Collect a continuous increment from the entire thickness of the exposed layer by scraping soil from the sidewall of the put. Ensure that an appropriate mass of soil is included in each increment to meet the targeted bulk sample mass.


Trenches can be strategically placed within a DU to investigate the presence of buried debris and evaluate the soil stratigraphy as well as collect MI samples (Figure G-9). In the example, targeted DU layers were excavated in successive lifts and placed on plastic for field screening with a portable XRF. Initial soil removal was carried out based on a subsequent map of the extent and depth of contaminated fill material. The floor and sidewalls of the excavated area were then subdivided into DUs for the collection of confirmation samples.
In Figure G-10, a trench approximately three feet wide was excavated to a depth of three to five feet to look for buried debris, staining and other signs of contamination an access targeted DU layers for initial sample collection and identification of COPCs. A 50-increment sample was collected from the exposed face of each of two targeted DU layers. Increments were collected in a systematic, random fashion across the entire extent of the DU layer (black bar in upper DU layer depicted in Figure G-10).
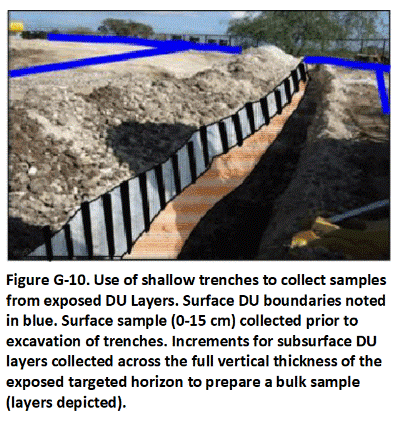
Field replicates can be collected within the same trench to assess the overall precision of the sample collection method. Independent sets of trenches within select DUs could be used to collect replicate samples in the same manner as done for surface samples and further assess the precision of the data.
Safety precautions are imperative to protect workers collecting samples during trenching. Excavations over 5 feet in depth typically need to be shored or properly sloped to prevent collapse during sample collection. A backhoe can be used to collect increments from the sidewalls of deep or otherwise unstable excavations.
Return to the Top of the Page
G.5 HOLLOW STEM AUGERS
Hollow-stem augers were already in use for drilling and coring in unconsolidated soils for geotechnical work at the advent of environmental investigations in the 1980s (Figure G-11 and G-12). The use of auger rigs is described in ASTM Standard D 5782 (ASTM 2006; see also USACE 2001; Nielsen 2006). The rigs are capable of reaching depths of 30 m (33 yd) in unconsolidated to semi-consolidated soil and even gravel, but cannot normally penetrate bouldery formations or lithified rock.
The use of auger rigs to collect soil samples has largely been replaced by more compact and efficient push rigs described above, although they are still frequently used for the installation of large-diameter monitoring wells. Hollow stem augers are not recommended for the collection of MI soil samples due to the large volume of soil removed at individual boreholes. The collection of samples from cuttings brought to the surface by the auger can be a useful part of exploratory boreholes, although it can be difficult to know the exact depth that the soil originated from. Layers of heavy contamination can also become mixed within the cuttings and obscured from observation.Return to the Top of the Page
G.6 ROTARY DRILLS
A detailed discussion of air or mud rotary drilling is beyond the scope of this technical guidance. Rotary drilling is generally used for geotechnical studies or the installation of wells through bedrock rather than for collection of soil samples (Nielsen 2006; US Navy 2015). These rigs are less useful for the collection of subsurface soil samples to be tested for contaminants. Aside from the expense and space required to operate the rigs, complete recovery of cores during drilling is difficult when drilling in unconsolidated and semi-consolidated lithologies, such as clays, silts, and sands. The rigs are most useful for the collection of continuous rock cores for geologic or geotechnical studies. Standard rock coring methods are summarized in ASTM guide D 2113 (ASTM, 2008). Standard rotary drilling methods are summarized in ASTM guide D 5782 (ASTM, 2006b).
APPENDIX H. COLLECTION OF EXCAVATION AND STOCKPILE SAMPLES
Return to the Top of the Page
H.1 EXCAVATIONS
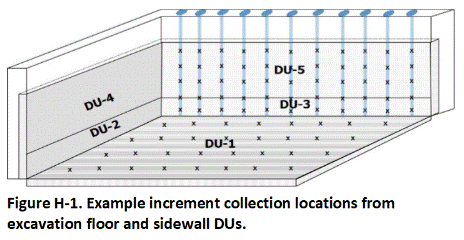 Multi Increment (MI) samples are collected from the walls and floor of an excavation in the same manner as done for exposed, surface soils (Figure H-1). Each Decision Unit (DU) represents a thin layer of soil in the walls and beneath the floor. As a default, assume a 10 to 15 cm (4-6 in) DU thickness for sample collection. Collect replicate samples from the floor or sidewall DU at highest risk of residual contamination (see Appendix L). The number of increments collected to prepare a sample should be pertinent to the type of contamination present (e.g., 30 to 75+; refer to Section 3.6.2). Ensure a minimum sample mass of 1 to 3 kg for samples to be tested for non-volatile chemicals. Sample collection methods for volatile chemicals are discussed in Appendix I.
Multi Increment (MI) samples are collected from the walls and floor of an excavation in the same manner as done for exposed, surface soils (Figure H-1). Each Decision Unit (DU) represents a thin layer of soil in the walls and beneath the floor. As a default, assume a 10 to 15 cm (4-6 in) DU thickness for sample collection. Collect replicate samples from the floor or sidewall DU at highest risk of residual contamination (see Appendix L). The number of increments collected to prepare a sample should be pertinent to the type of contamination present (e.g., 30 to 75+; refer to Section 3.6.2). Ensure a minimum sample mass of 1 to 3 kg for samples to be tested for non-volatile chemicals. Sample collection methods for volatile chemicals are discussed in Appendix I.
A push rig can be used to collect confirmation samples prior to excavation, as depicted for the rear wall of the excavation in the figure. This can negate the need for follow-up testing and expedite project completion if screening levels are met and the samples meet minimum increment spacing and bulk sample mass requirements. Increments should be collected in an evenly spaced, systematic random manner both within cores and between adjacent cores (refer to Section 3.6.2). This might require the installation of very closely spaced borings for a sidewall DU that is significantly longer than it is tall (sidewalls) or wide (floors), since a minimum of 30 borings would be required. Refer to Appendix F for additional guidance on the collection of samples from long, narrow DUs. Follow-up collection of confirmation samples from exposed sidewalls after excavation is required if the collection of high-quality samples prior to removal of soil is not possible.
Return to the Top of the Page
H.2 STOCKPILES
It is important to ensure that soil from different sources or with suspected different magnitudes of contamination is kept segregated. This will help minimize accidental mixing of contaminated soil with clean soil and expedite testing of the soil and determination of options for reuse or disposal. Similar approaches can be used to segregate and characterize mine tailings, dredged sediment, biosolids and other types of particulate media. Additional guidance on testing of stockpiles or other sources of material to be used as fill material is provided in Appendix P.
The general approach to characterization of stockpiled soil include:
- Segregate stockpiles with respect to different source areas;
- Further segregate the soil into anticipated clean and anticipated contamination stockpiles;
- Select appropriate DU volume(s) based on proposed reuse of soil land use and contaminants of concern;
- Choose a sampling strategy and tools that will provide access to sampling points throughout each DU;
- Collect triplicate MI samples in 10 percent of DUs (minimum one set; see Appendix L);
- Consider the specific timing of the sampling activities – sampling during stockpile formation is ideal but might not be practical from a safety standpoint.
Table I-1 summarizes the DU volume recommended for characterization of fill material in stockpiles. Decision units for stockpiles should generally be designated in terms of volume, rather than area. The appropriate DU volume for a stockpile is based on several factors, including:
- Targeted contaminants and associated environmental hazards;
- Proposed use of fill material at receiving site (e.g., residential versus commercial or industrial property, etc.); and
- Assumed reuse of soil and potential exposure areas and soil placement thickness;
- Total volume of fill material to be characterized.
Allowances for larger DU volumes can be made for deeper material not known or suspected to be contaminated (e.g., native soil) as well as sediment to be dredged from areas that lack known sources of potentially significant contamination, where testing is primarily for due diligence purposes. Final DU volume limitations should be discussed on a case-by-case basis with the overseeing regulatory agency.
Highly volatile or leachable chemicals can pose significant vapor intrusion or leaching hazards that could require a more detailed characterization of the proposed fill material. Small DU volumes are recommended for testing of soil suspected to be contaminated with these types of chemicals. The recommended volume to a single DU for reuse at residential sites, schools, parks and commercial or industrial facilities is based in part on the assumed thickness of the material to be placed in an area and the size of exposure areas typically assessed as part of a risk assessment (refer to Section 3.3.4 and Appendix C). Volume is calculated by multiplying the size of the assumed exposure area by the assumed thickness of the fill material to be placed at a site.
While somewhat subjective, the recommended range of default stockpile DU volumes serves as a useful starting point for discussions and can be made more case specific as needed. Be aware that placement of thick units of fill material at a site and corresponding characterization of very large volumes of material as a single DU could place an inherent restriction on future reuse of the soil at other sites in the absence of additional testing.
It is important to allow equal access to all soil within a stockpile DU for the collection of increments. Increments collected from only the exposed surface of a stockpile, for example, might not be representative of deeper soil. When space is available, the stockpile should be flattened to a thickness of three feet or less to allow equal access to all soil in the pile (Figure H-2). Increments are then collected from the top, middle, and bottom of the pile in a systematic random fashion.
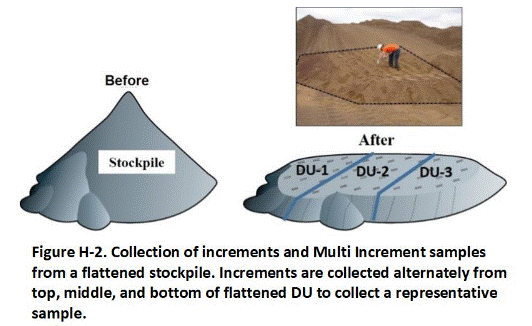
Another option is to collect increments as the stockpile is being formed (Figure H-3). For example, increments could be collected from front-end loader buckets at appropriate intervals of soil volumes as the soil is being excavated or moved. Increments could also be collected as piles are formed at the end of a conveyor belt, or when stockpiles are being moved from one location to another. This allows equal access to each portion of the pile as it is constructed, avoids the need to reconstruct a stockpile, and saves space required to flatten a stockpile for sampling. The collection of soil samples during these activities can interfere with the operation of heavy equipment, pose a risk to the sample collector and requires careful coordination between all parties.
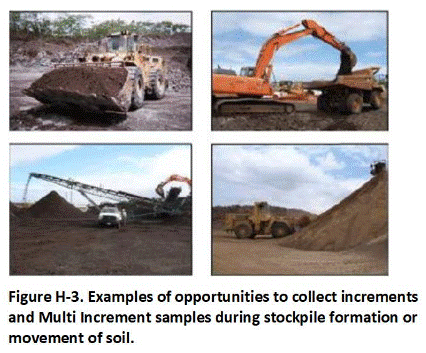
A third option for stockpiles that cannot be spread out is to progressively characterize accessible soil from one or more faces of the pile as the soil is needed (Figure H-4). Collected increments in a systematic, random fashion from the targeted volume of soil in a manner that meets minimum increment spacing and bulk sample mass requirements. Ensure that the targeted volume of soil does not exceed recommended limits noted in Table H-1 or as otherwise approved by the overseeing regulatory authority. Alternative DU volumes can be proposed and supporting information provided on a case-by-case bases. Soil represented by the resulting sample can be cleared for reuse or disposed as appropriate. Replicate samples could be collected from every tenth DU volume of soil tested to verify the adequacy of the overall sampling method (see Appendix L).
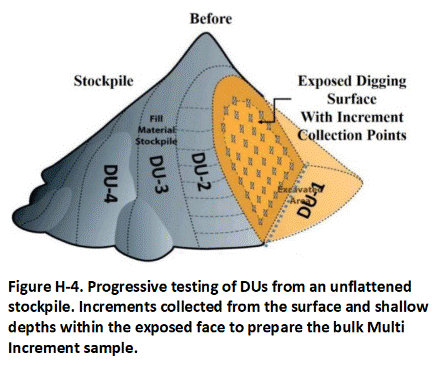
In some cases, testing of very large existing stockpiles using one of the above-noted methods might not be possible due to the lack of space and a need to test the soil for immediate removal from the site. In such cases, divide the stockpile into DUs based on the recommended volume limits noted in Table H-1 or as otherwise approved and mark the boundaries of DUs on the surface of the stockpiles. Collect a sample from the exposed surfaces of the designated DUs in accordance with standard MI sampling procedures for surface samples (refer to Appendix F). Use a backhoe or other equipment to dig pits into deeper parts of as many DUs as possible. Inspect the soil for changes in soil type, color, debris, staining, odors and other indications of potential contamination. Collect at least one sample from the entire exposed area of the excavation. Compare this data to data for the surface sample from the same DU. Consider limitations in the data due to the inability to access all parts of the pile and the known or suspected source of the soil when determining appropriate reuse or disposal.
Table H-1. Example default stockpile DU volumes based on targeted contaminants of concern and receiving site land use.
| Targeted Contaminants of Concern | Receiving Site Land Use Category | 1Default Fill Reuse Area and Thickness | 2,3Default Stockpile Decision Unit Volume | Example COPCs | Primary Potential Environmental Hazards |
| 4Volatile Compounds | Any | (not applicable) | 100 yds3 | TPHg, TPHd, BTEX, naphthalene, PCE, TCE, mercury | Vapor intrusion, leaching |
| 4,5Highly Leachable, Non‐Volatile Contaminants | Any | (not applicable) | 100 yds3 | chlorinated herbicides, perchlorate, PFASs, explosives | Leaching and surface runoff or groundwater contamination |
| 5,6,7Low Mobility Contaminants | Unrestricted Use | Area: 5,000 ft2 Thickness: 6 in |
100 yds3 | heavy metals, dioxins/furans, PCBs, PAHs, TPHo, organochlorine pesticides | Direct exposure |
| Schools and High‐Density Residential Developments | Area: 20,000 ft2 Thickness: 6 in | 400 yds3 | |||
| 8Parks and athletic fields (contamination) | Area: 20,000 ft2 Thickness: 6 in | 400 yds3 | |||
| 9,11,12Commercial/Industrial use only (localized fill source from previously developed area; assumed low-moderate risk of contamination) | Area: 20,000 ft2 Thickness: 6 in | 400 yds3 | |||
| 10,11,12Commercial or Industrial use only (large, agricultural field, undeveloped land or dredged sediment fill source assumed low risk of contamination) | Area: 40,000 ft2 Thickness: 6 in | 800 yds3 |
Notes:
COPCs Contaminants of Potential Concern
BTEX Benzene, Toluene, Ethylbenzene, and Xylenes (total)
PAHs Polycyclic Aromatic Hydrocarbons
PCBs Polychlorinated Biphenyls
PCE Perchloroethylene
PFASs Perfluoroalkyl substances
TCE Trichloroethylene
TPHg Total Petroleum Hydrocarbons as gasoline
TPHd Total Petroleum Hydrocarbons as diesel
TPHo Total Petroleum Hydrocarbons as heavy oil
ft2 square feet
cyds cubic yards
- Placement area and thickness of fill material reflects hypothetical exposure areas for noted land-use scenarios; used to derive target DU volumes for individual testing (rounded). DU area size of 40,000 ft2 used to approximate one-acre. DU areas for isolation of suspected source areas and optimization of remedial actions might be smaller than default areas and volumes noted (refer to Section 3.2.2).
- Noted DU volumes reflect rounding of associated areas and thicknesses of soil. Isolate known or suspected, heavily contaminated volumes of soil for treatment or disposal to the extent practicable. Larger volumes might be acceptable on a case‐by‐case basis. DU volume up to 400 yds3 acceptable for unrestricted reuse, including single-family homes, schools and high‐density residential developments on case-by-case basis if prior knowledge and a thorough Phase I indicates low potential for contamination. DU volume up to 800 yds3 acceptable for parks and athletic fields and commercial or industrial sites based on same rational. Using DU sizes larger than accepted for unrestricted fill material reuse for commercial/industrial properties might require retesting of property where fill material is placed if property is proposed for more sensitive land use in the future (e.g., residential). Allowances for larger DU volumes can be made for deeper, native soil or dredged sediment from areas that lack known sources of potentially significant contamination, where testing is primarily for due diligence purposes. Consideration of a “fluff factor” and larger volumes can be made for stockpiled ex situ soil that will be compacted during use as fill material.
- Collect triplicate MI samples in 10% of DUs (minimum one set).
- Minimum 1.5 ft thickness of fill material assumed necessary to contain sufficient contaminant mass to pose long-term vapor intrusion or leaching risks (based on finite mass vapor intrusion models of common VOCs). Review on a site-specific basis as needed. Appropriate DU volume for highly volatile or highly leachable chemicals is site-specific and depends in part on the mass of the contaminant present. Collect soil vapor data to assess vapor intrusion risks for existing or future buildings.
- Assumed area reflects default range of direct-contact, exposure areas utilized in risk assessments. Minimum 0.82 ft (0.25 m) thickness of fill material assumed placed at reuse sites and used to calculate range of default DU volumes.
- Include laboratory batch leaching tests and/or soil column leaching tests to risk posed by leaching of metals if contamination above unrestricted, direct-exposure screening levels is to be left in place and reliable, leaching based screening levels or if screening levels are not available (HIDOH 2024).
- Using soil with known pockets of low volatility and relatively immobile heavy oil as fill material not recommended due to gross contamination concerns (odors, staining, sheens in runoff, etc.) and public perception concerns.
- Residential/Unrestricted Land Use screening levels and target risks apply.
- Assumed localized fill source from previously developed area with low to moderate risk of contamination.
- Assumes large fill source from agricultural land, undeveloped land or sediment dredged from areas with no know past or current source of pollutants and minimal risk of contamination. Thorough Phase I review of historical site use required and used to support larger DU volumes and, if adequate, could negate need for testing other than for due diligence purposes. Maximum DOH volume of 1,600 yds3 recommended (assumes used as fill material for one-acre area with a thickness of one foot.
- Commercial/Industrial screening levels and target risks apply.
- Noted DU volumes also suitable to testing of soil or dredged sediment for daily/interim/intermediate/final landfill cover. Refer to Clean Fill Guidance (Appendix P, HIDOH 2017).
APPENDIX I. Sample Collection for Volatile ContaminantsReturn to the Top of the Page
I.1 OVERVIEW
Refer to Table I-1 for a list of example chemicals considered to be volatile. Tables I-2 and I-3 list example chemicals considered to be semi-volatile or otherwise chemically unstable. A discussion of laboratory processing of samples to be tested for the latter is provided in Appendix K, Section K-6.
Samples to be tested for volatile organic compounds (VOCs) are most commonly collected from cores extracted from targeted subsurface Decision Unit (DU) layers, sidewalls and floors of excavations or stockpiles of soil suspected to be contaminated with fuels or solvents. Testing of soil and sediment for semi-volatile or otherwise unstable compounds is discussed under laboratory processing in Appendix K (refer to Section K-6). Testing of samples from freshly exposed faces of excavations for VOCs might be required to confirm removal of contaminated soil. Testing of exposed shallow surface soil (e.g., 1 to 15 cm (0.-6 in)) for VOCs is usually not warranted due to the anticipated loss of volatile compounds to ambient air. Exceptions include characterization of recent spills or historical heavy contamination of surface soil. Soil vapor samples should be collected at sites where there is a potential risk of vapor intrusion into existing or anticipated future buildings (HIDOH 2021).
The collection of soil samples to be tested for VOCs is similar to that described for non-volatile contaminants, except that increments or subsamples of increments from cores are placed in methanol or an alternative extraction solution in the field. Alternative methods for combining and/or preserving increments are discussed later in this section. The collection of samples to be tested using this method should be discussed with the laboratory well in advance of field work. The analytical laboratory should be consulted prior to sample collection to discuss sample containers, sample handling, preservative type and volume, shipping of samples in methanol, anticipated laboratory method detection limits, etc.
Laboratory Method 5035 provides options for preservation of samples based on desired detection limits and desired holding time limitations (USEPA 2002h; refer also to MADEP 2002, TRNCC 2002 and CAEPA 2004b). The best option in terms of data representativeness is to combine increments in a container with methanol. The amount of methanol placed in the container is calibrated to the mass of soil increments anticipated to be collected for a specific sample. Tools that extract core-shaped plugs of soil are utilized in the field to ensure a consistent mass of individual increments and to approximate the total mass of material collected. Coring devices with calibrated sample collection volumes are generally utilized so reasonable estimates of total mass can be made. The total mass of soil placed in the solution should closely match the mass initially relayed to the laboratory to ensure the soil remains covered by methanol during sample storage and shipment. The laboratory estimates the original concentration of the targeted VOC in soil by dividing the mass of the VOC estimated to be in the methanol by mass of soil placed in the container.
The use of methanol under Method 5035 allows for a holding time of 14 days prior to analysis by the laboratory. Ideally, samples should reach the laboratory within 48 hours of collection to verify that methanol is not being lost from the container. Methanol loss would introduce error into the calculation of the original concentration of the VOC in the soil sample. Potential problems with the use of methanol include an increase in method detection levels due to the need to dilute the solution for analysis and logistical issues related to obtaining, storing and shipping the flammable solution. These issues should be resolved with the laboratory prior to the collection of samples in the field.
Although allowed as an option under Method 5035, the use of reagent-grade water rather than methanol as an extraction solution is not recommended. This approach was included in the lab method to allow for lower detection limits in comparison to samples extracted into methanol (USEPA, 2002h). Improvements in laboratory methods since that time should provide methanol-based reporting limits of 50 µg/kg for volatile chemicals, which is more than adequate for screening purposes.
Water-based extraction is also significantly less effective in comparison to methanol according to laboratories contacted by the overseeing regulatory agency. While the precision of the data in terms of the analytical method might be higher than for methanol, this is likely to be outweighed by error associated with incomplete extraction of VOCs from the soil as well potential degradation as during storage and shipment. In addition, VOCs will be less tightly held in water than in methanol and can be lost when the sample bottle is opened repeatedly to add increments. A further disadvantage is that samples must either be analyzed within 48 hours or frozen to -7°C within 48 hours and then analyzed within seven days from the sample collection date. This limited holding time can pose additional problems for samples that must be shipped for analysis.
The use of an acidic sodium bisulfate solution as an alternative to water is also provided under Method 5035. It provides both an extended holding time (up to 14 days) and allows for very low detection levels. Reaction with organic matter, effervescence and loss of VOCs in calcareous soils, and other potential problems interfere with the practical use of this approach in the field. Use of a sodium bisulfate solution is not recommended unless a site-specific field study is carried out to demonstrate the data are comparable to using a methanol-based solution.
Note that soil data for volatile compounds are most efficiently used to estimate the mass of the contaminant present within a DU as a tool to assist in the design of remedial efforts. The primary environmental risks associated with volatile chemicals are vapor intrusion into overlying buildings and/or leaching of the contaminant into underlying groundwater. In both cases, the collection of soil gas sample data can serve as more directly applicable alternative to soil sample data, provided that soil gas-based action/screening levels are available or can be developed for comparison (refer to Section 13 of the TGM; see also HIDOH 2017).
I.2 INCREMENT COLLECTION AND SAMPLE PREPARATION
A volume of methanol, adequate to accommodate the estimated total mass of increments to be collected for a sample, is placed in the sample bottle prior to collection of the sample. A minimum 1:1 ratio of solution volume to soil mass is recommended (i.e., 1 ml of methanol to 1 gram of soil). This generally allows for slightly more methanol to be in the container by volume than soil and ensures that the soil remains saturated and covered with methanol during storage and shipment.
The laboratory will typically provide sample jars with pre-measured amounts of the solution based on direction from the sampler and regarding the approximate mass of soil to be added. The addition of methanol in the field might be required to ensure the sample mass is completely submerged. The specific volume of methanol added should be documented and discussed with the laboratory that will receive and analyze the samples, since calculation of the original concentration of the VOC relies on accurate knowledge of the volume of methanol placed in the sample container. Increasing the volume of methanol in the container will lower the reporting limits for VOCs but is necessary to ensure data representativeness.
To select the appropriately sized sample container, consideration should be given to the total volume of soil to be collected and the volume of preservative required. A minimum 300 gram mass of soil should be collected to prepare a bulk Multi Increment (MI) sample. For example, 60 increments of 5 grams each for a total of 300 grams of soil (minimal recommended sample mass) would require approximately 300 ml of preservative. Utilize a container that is large enough to accommodate additional preservative (if needed) and to prevent loss of preservative through splashing as soil increments are dropped into the container. This can normally be accomplished using one-liter, amber glass bottles pre-filled at the laboratory with 300 ml of methanol.
Shipping constraints might require placement of increments in multiple small viles for combination at the laboratory. Methanol-preserved samples should be stored in a cool location away from direct sunlight until they can be placed in a cooler or refrigerator. Methanol should never be stored in a refrigerator. It is a clear, odorless, flammable liquid and could produce an explosion in a refrigerator. Only store methanol in coolers with ice or gel ice.
It is very important to remember that samples to be tested for VOCs be placed in methanol immediately after collection to prevent potential loss due to volatilization and/or biodegradation. Good planning of the field sampling effort is essential to ensure that reliable VOC sample data are collected appropriately.
Return to the Top of the Page
I.3 SUBSURFACE DU LAYERS
Each section of core extracted from a targeted DU layer represents an increment for that layer in the same manner that a small core extracted from a surface DU layer represents a single increment for that layer (see Appendix G). These are referred to as “core increments.” Individual core increments extracted from borings can be too large for combination into a manageable sample, however, and will normally need to be subsampled in the field to prepare a final, bulk sample.
Identify targeted DU layers in a core increment immediately after the core is received for sample collection. Evenly spaced plugs of soil should be removed from the core increment and placed in a container specifically designated for that DU layer (Figure I-1). The container should be pre-filled with a volume of methanol appropriate for the anticipated final mass of soil to be collected, normally a one-to-one ratio of methanol volume to soil mass (see Section I.2). The use of plugs rather than wedges helps control the total mass of soil collected and minimize disturbance of the soil during collection. Subsamples from core increments from other borings installed into the same DU layer are progressively added to the container specific to that layer as the field investigation advances to prepare a final sample.
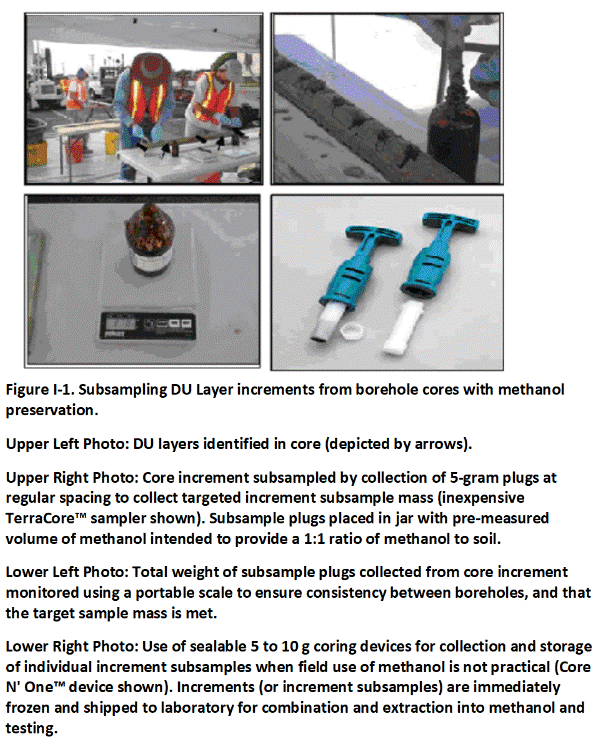
It is very important that sample increments be placed in methanol as soon as possible (i.e. within a minute or two) to prevent potential loss of volatiles due to volatilization and/or biodegradation. Good planning of the field sampling effort is essential to help ensure VOC samples are collected appropriately.
The total mass of increment subsamples should be adequate to prepare a minimum, 300-gram sample. A smaller mass of soil is generally acceptable for the collection of a sample to be tested for VOCs due the assumed absence of high concentration “nuggets” of contaminated soil and a greater degree of compositional heterogeneity associated with non-liquid and non-volatile contaminants (see Appendix D). If the thickness of the DU layer is anticipated to be consistent across the DU area, then divide the target mass of the final sample by the number of core increments to be collected to determine that total subsample mass that should be collected from a single increment. For example, collection of a 300-gram sample from 30 boreholes would require that ten grams of soil e.g., two five-gram plugs) be collected from each core increment.
Space the subsample plugs in a manner that covers the total thickness of the core increment as efficiently as possible. Note that the collection of smaller subsample plugs allows for more efficient coverage of an individual increment, since the collection of more plugs is required to meet the target sample mass.
Collection of a targeted mass of soil from a DU layer that varies in thickness requires more advanced planning. The same sample mass will be collected, but the mass of subsamples collected from individual increments will necessarily vary to bias the mass of the final sample toward the thicker areas of the layer. Approximate the average thickness of the layer based on data from exploratory boreholes (see Appendix G). Multiply this thickness by the number of core increments to be collected. This represents the total length of the combined core increments. Divide the target final sample mass by the mass of a single subsample plug. For example, preparation of a 300-gram sample based on combination of 5-gram subsample plugs requires the collection of 60 plugs.
Divide the total length of the combined core increments by the number of subsample plugs to be collected. The result reflects the target spacing of plugs to be extracted from any individual core increment. Maintain consistent plug spacing for subsampling of all core increments collected from the DU layer. This will help ensure that the final sample is properly weighted toward thinner and thicker areas of the layer, since more plugs will necessarily be collected in thicker areas.
When sampling VOCs in soils it is important to ensure that soil is placed in the preservation fluid (methanol) within a few minutes after collection to reduce losses due to volatilization and biodegradation. This can be especially challenging when collecting samples or subsamples from multiple subsurface borings and multiple DU layers per boring. Very close coordination is necessary between the drill crew and the sampling crew to minimize the time between extraction of the core and placement of increment subsamples in methanol.
Set up a well-organized workstation for processing the subsamples (see Figure I-1). All sample containers should be pre-labeled to save processing time. Adequate storage containers/coolers with ice should be readily available as samples are prepared. If a nearby indoor workstation is not available for use, then a field workstation with tarps or covers for rain, sun, and/or wind protection might be needed. A minimum of two people is normally required to efficiently prepare samples and minimize delays on the collection of cores by the drill team. One person should be assigned to each targeted DU layer to avoid inadvertent mixing of subsamples between cores.
Increments should be collected using tools that minimize the loss of volatile chemicals during sample collection (e.g., cause the least disaggregation of soil) and allow the collection of at least a five-gram plug of soil. Syringe-type, core-shaped devices that can be pushed directly into the soil are preferable. Examples include the TerraCore™, Core N’ One™ and Encore™ tools (see Figure I-1). Inexpensive, plastic, disposable syringes with the forward ends cut off are convenient for subsample collection when methanol can be used in the field. These types of devices can also be used for the collection of subsamples from core increments.
As depicted in Figure I-1, the device is pushed into the soil, retracted, and the increment collected is immediately extruded into a container with a premeasured volume of preservative (e.g., methanol). Then end of device can be trimmed to make a scoop for subsampling of gravelly soils. This is repeated with each increment or increment subsample. Dedicated sampling devices should be used between different DU layers within a single borehole but can be reused for subsampling of core increments in multiple DU boreholes for the same DU layer.
A single large jar with a pre-measured volume of methanol adequate for the entire targeted DU layer could in theory be used to prepare a bulk MI sample in the field (i.e., plugs from subsample increments combined from 30+ borings). However, this risks an expensive total loss of the sample should the jar be accidentally broken in the field or at the laboratory.
An alternative approach is to place subsample plugs for individual core increments into a smaller jar specific to each borehole. Methanol from individual jars (aliquots) representing the same targeted DU layer can then be combined at the laboratory for testing. This approach can also allow for different vertical and lateral combinations of core increments to be evaluated to obtain a better resolution of the location of the core mass of contamination at depth and help optimize remediation.
Subsample replicates should be collected from 10% of the borings and compared to evaluate the precision of method used. This will involve the collection and combination of three separate sets of subsample plugs from the same boring for each of the targeted DU layers. The relative standard deviation of the replicate data sets should be compared and the subsampling method modified, as needed, to achieve an acceptable precision. Increasing precision could require a decrease in plug spacing and the collection of more plugs per DU layer interval and/or an increase in the mass of subsamples collected from individual increments.
Return to the Top of the Page
I.4 SAMPLE SHIPMENT
Methanol is a hazardous material with flammability and toxicity concerns. Check with local regulations and the carrier to be used for shipping requirements. The maximum-allowed, “Excepted” quantity for air shipping of methanol in many countries is no more than 30 milliliters per inner container and a maximum of 0.5 liter per cooler or package. There is no limitation on the number of separate coolers or packages that can be shipped as long as each individual cooler meets the methanol quantity limits. Confer with airline used and applicable International Air Transport Association regulations. Ensure that the package(s) are properly labeled and the shippers have knowledge regarding the samples and sampling methods used. Check with the shipper beforehand to ensure that containers are properly labeled and shipping requirements met. Restrictions could vary between islands and airlines. For shipping methanol above the excepted quantities, a hazmat-trained shipper or packer should be utilized, or someone with equivalent training/certification and knowledge of applicable regulations.
Return to the Top of the Page
I.5 ALTERNATIVES TO METHANOL PRESERVATION
Collection of Methanol Subsamples
Alternatives can be considered in consultation with the laboratory in cases where volumes of methanol greater than 30 milliliters per container presents problems for air shipping. For example, place MI sample increments into the full, recommended volume of methanol in the field. Record the exact volume of methanol and total mass of soil placed in the container and provide this information to the laboratory. Agitate the sample and allow the solution to equilibrate over a twenty-four-hour contact period. Decant at least 20 ml of the solution into a standard 40 ml VOA vile using a gas-tight syringe (check with laboratory on required volume).
This alternative should only be conducted under a specific procedure provided by the laboratory and is included in the site investigation report. Ship the samples to the laboratory under the “Excepted” quantity category for methanol (in accordance with airline used, and applicable IATA regulations). Method 5035A also notes that sonification of samples at 40°C for 30 minutes can be carried out for samples with less than 24 hours contact. If this option is available, then it can be used to shorten methanol contact time required before subsampling and shipping samples by air.
Note that the remaining spent methanol mixture is classifiable as a listed hazardous waste under some regulations and must be managed accordingly (e.g., classification as F003 waste for spent, non-halogenated solvents under Section 261, Title 40 of the USEPA Code of Federal Regulations). The spent mixture might also be classifiable as a hazardous waste due to ignitability. However, quantities of waste methanol generated will likely be minimal, in which case regulations for conditionally exempt small quantity generators will apply (e.g., 100 kg limit in USEPA regulations).
A potential limitation of the extraction of samples in methanol is an increase in method detection limits (MDLs). This could cause the MDLs to be above relevant screening levels for certain targeted chemicals. MI soil samples for volatile analyses can be tested using Selected Ion Monitoring (SIM) laboratory methods to reduce method reporting limits to target action levels for samples preserved in methanol. The SIM methods target a small number of select compounds instead of a full standard VOC list, and typically allow an order-of-magnitude reduction in reporting limits in comparison to standard Method 8260 analysis. If problems persist, then the investigation objectives should be reviewed and discussed with the overseeing regulatory agency. High-quality sample data with elevated detection limits are preferable to low-quality data with lower detection limits.
Freezing Increments
Although not ideal, sample increments can be frozen immediately after collection and shipped to the laboratory for combination in methanol (USEPA, 2002h). This might be unavoidable due to the logistical difficulties of obtaining, storing, and shipping methanol to a laboratory.
Sample increments should be stored in individual devices constructed specifically for this purpose that have vapor tight seals and are designed for zero headspace (e.g., Core N’ One™, EnCore™, or equivalent type sampler; see Figure I-1). An alternative is to place multiple increments into a vapor-tight jar provided by the laboratory. This risk disturbance of the increments however, and loss of VOCs.
Increments should be immediately frozen in the field to between -7°C and -15°C if possible. This approach provides for a 14-day holding time but is not as reliable as methanol extraction in the field. Prepare ice mixed with saltwater ice to achieve the target temperature range (e.g., mix 25 grams of sample for every 100 grams of ice; Hewitt, 1999). If dry ice is utilized, then packing methods should be placed between the sample containers and the ice to avoid damage to the containers or seals. Dry ice can achieve a temperature of -40°C and can cause severe damage to skin if touched. Check with the shipper for specific procedures, including the amount of dry ice that can be placed in a single cooler, package labeling, and requirements for the use of a vented cooler. Dry ice is not normally allowed on commercial flights.
If immediate freezing of the increments is not possible, the store the containers on normal ice between 2°C and 6°C and submit them to the laboratory within 48 hours for combination and extraction in methanol. This approach has the highest risk of VOC loss and non-representative data error and should be avoided if possible.
Collection of Soil Gas Samples
Soil sample collection methods described above might not be feasible for example investigations to be carried in remote areas where transport and storage of methanol is precluded due to logistical or safety reasons or keeping samples frozen in the field is similarly not possible. Soil gas sample data can be used as an alternative for assessment of risk. The primary risks associated with volatile chemicals are vapor intrusion into overlying buildings and/or leaching of the contaminant into underlying groundwater. In both cases, the collection of soil gas sample data can serve as a more directly applicable alternative to soil sample data. Soil gas-based action levels for vapor intrusion risk and leaching concerns are provided in the HIDOH EAL guidance (HIDOH 2017; refer also to Section 13 of the TGM). Direct testing of groundwater can serve as an alternative to use of soil gas action levels for leaching concerns if practicable.
Note that neither soil gas nor groundwater data are reliable for estimation of the total mass of a volatile chemical present in soil. This is because the majority of the mass is likely to be sorbed to organic carbon and clay in the soil itself. Use of simplistic equilibrium partitioning models to predict the sorbed concentration and mass can significantly underestimate the actual mass of contaminant present and lead to failure of in situ remedial actions.
TABLE I-1 Volatile Chemicals Requiring Field Preservation of Soil Sample Increments
Reference: Appendix 1, Table H in HEER Office Environmental Hazard Evaluation guidance (HDOH 2017 and updates).
| 2Vapor Pressure | Henry’s Law Constant (H) |
||||
| CHEMICAL PARAMETER | 1Physical State | Molecular Weight | mm Hg (25C) | (atm-m³/mol) | |
| VOLATILE CHEMICALS Preserve Samples in Methanol in the Field (or approved alternative, see text) (VP>1 and Molecular Weight <200) |
|||||
| ACETONE | V | L | 58 | 2.3E+02 | 3.9E-05 |
| BENZENE | V | L | 78 | 9.5E+01 | 5.61E-03 |
| BIS(2-CHLOROETHYL)ETHER | V | L | 143 | 1.6E+00 | 1.7E-05 |
| BROMODICHLOROMETHANE | V | L | 164 | 5.0E+01 | 2.1E-03 |
| BROMOFORM | V | S | 253 | 5.4E+00 | 5.4E-04 |
| BROMOMETHANE | V | G | 95 | 1.6E+03 | 6.3E-03 |
| CARBON TETRACHLORIDE | V | L | 154 | 1.2E+02 | 2.7E-02 |
| CHLOROBENZENE | V | L | 113 | 1.2E+01 | 3.2E-03 |
| CHLOROETHANE | V | G | 65 | 1.0E+03 | 1.1E-02 |
| CHLOROFORM | V | L | 119 | 2.0E+02 | 3.7E-03 |
| CHLOROMETHANE | V | G | 50 | 4.3E+03 | 8.8E-03 |
| CHLOROPHENOL, 2- | V | L | 129 | 2.5E+00 | 1.1E-05 |
| DIBROMOCHLOROMETHANE | V | S | 208 | 5.5E+00 | 7.8E-04 |
| DIBROMOETHANE, 1,2- | V | S | 188 | 1.1E+01 | 6.6E-04 |
| DICHLOROBENZENE, 1,2- | V | L | 147 | 1.4E+00 | 1.9E-03 |
| DICHLOROBENZENE, 1,3- | V | L | 147 | 2.2E+00 | 1.9E-03 |
| DICHLOROBENZENE, 1,4- | V | S | 147 | 1.7E+00 | 2.4E-03 |
| DICHLOROETHANE, 1,1- | V | L | 99 | 2.3E+02 | 5.6E-03 |
| DICHLOROETHANE, 1,2- | V | L | 99 | 7.9E+01 | 1.2E-03 |
| DICHLOROETHYLENE, 1,1- | V | L | 97 | 6.0E+02 | 2.7E-02 |
| DICHLOROETHYLENE, Cis 1,2- | V | L | 97 | 2.0E+02 | 4.1E-03 |
| DICHLOROETHYLENE, Trans 1,2- | V | L | 97 | 3.3E+02 | 9.3E-03 |
| DICHLOROPROPANE, 1,2- | V | L | 113 | 5.3E+01 | 2.9E-03 |
| DICHLOROPROPENE, 1,3- | V | L | 111 | 3.4E+01 | 3.7E-03 |
| DIOXANE, 1,4- | V | L | 88 | 3.8E+01 | 4.9E-06 |
| ETHANOL | V | L | 46 | 5.9E+01 | 6.3E-06 |
| ETHYLBENZENE | V | L | 106 | 9.6E+00 | 7.8E-03 |
| METHYL ETHYL KETONE | V | L | 72 | 9.1E+01 | 5.6E-05 |
| METHYL ISOBUTYL KETONE | V | L | 100 | 2.0E+01 | 1.4E-04 |
| METHYL TERT BUTYL ETHER | V | L | 88 | 2.5E+02 | 5.9E-04 |
| METHYLENE CHLORIDE | V | L | 85 | 4.4E+02 | 3.2E-03 |
| STYRENE | V | L | 104 | 6.4E+00 | 2.7E-03 |
| tert-BUTYL ALCOHOL | V | L | 74 | 4.1E+01 | 1.2E-05 |
| TETRACHLOROETHANE, 1,1,1,2- | V | L | 168 | 4.6E+00 | 2.4E-03 |
| TETRACHLOROETHANE, 1,1,2,2- | V | L | 168 | 4.6E+00 | 3.7E-04 |
| TETRACHLOROETHYLENE | V | L | 166 | 1.9E+01 | 1.8E-02 |
| TOLUENE | V | L | 92 | 2.8E+01 | 6.6E-03 |
| TPH (gasolines) | V | L | 108 | 6.8E+02 | 7.2E-04 |
| TRICHLOROETHANE, 1,1,1- | V | L | 133 | 1.2E+02 | 1.7E-02 |
| TRICHLOROETHANE, 1,1,2- | V | L | 133 | 2.3E+01 | 8.3E-04 |
| TRICHLOROETHYLENE | V | L | 131 | 6.9E+01 | 9.8E-03 |
| TRICHLOROPROPANE, 1,2,3- | V | L | 147 | 3.7E+00 | 3.4E-04 |
| TRICHLOROPROPENE, 1,2,3- | V | L | 145 | 3.7E+00 | 2.8E-02 |
| VINYL CHLORIDE | V | G | 63 | 3.0E+03 | 2.7E-02 |
| XYLENES | V | L | 106 | 8.0E+00 | 7.1E-03 |
- Physical state of chemical at ambient conditions (V – volatile, SV – Semi-Volatile (*SV – Treated as “volatile” in USEPA risk assessment models if H > 0.00001), S – solid, L – liquid, G – gas).
- Vapor Pressures from National Library of Medicine TOXNET or ChemID databases.
- Check with lab to determine feasibility of wet sieving sample to remove >2mm particles prior to subsampling.
- Soil or sediment samples that consist entirely of <2mm material do not require drying and sieving to address fundamental error concerns, although some degree of drying and sieving may be desirable by the laboratory for testing purposes.
Table I-2 Semi-volatile or Otherwise Unstable Chemicals Requiring Laboratory Subsampling of Soil Samples Prior to Processing (refer to Appendix K, Section K.6)Reference: Appendix 1, Table H in HEER Office Environmental Hazard Evaluation guidance (HDOH 2017 and updates).
| CHEMICAL PARAMETER | 1Physical State | Molecular Weight | 2Vapor Pressure mm Hg (25C) | Henry’s Law Constant (H) (atm-m³/mol) |
|
| SEMI-VOLATILE AND OTHER SEMI-STABLE CHEMICALS 3,4Subsample Multi Increment Bulk Sample at Laboratory Upon Receipt Without Drying (VP 0.1 to 1.0 OR Liquid at 25C OR Henry’s Constant >1.0E-05) |
|||||
| BIPHENYL, 1,1- | *SV | S | 154 | 8.9E-03 | 3.2E-04 |
| BIS(2-CHLOROISOPROPYL)ETHER | *SV | L | 171 | 8.5E-01 | 1.1E-04 |
| DALAPON | *SV | L | 143 | 1.9E-01 | 9.0E-08 |
| DIBROMO,1,2- CHLOROPROPANE,3- | *SV | L | 236 | 5.8E-01 | 1.5E-04 |
| DIMETHYLPHENOL, 2,4- | SV | S | 122 | 1.0E-01 | 9.5E-07 |
| HEXACHLOROBUTADIENE | SV | S | 261 | 2.2E-01 | 1.0E-02 |
| HEXACHLOROETHANE | SV | S | 237 | 4.0E-01 | 3.9E-03 |
| ISOPHORONE | SV | L | 138 | 4.4E-01 | 6.6E-06 |
| 5MERCURY | *SV | S | 201 | 2.0E-03 | – |
| METHYL MERCURY | SV | S | 216 | – | – |
| NITROBENZENE | *SV | L | 123 | 2.5E-01 | 2.4E-05 |
| NITROGLYCERIN | SV | L | 227 | 2.0E-04 | 9.8E-08 |
| NITROTOLUENE, 4- | SV | S | 137 | 1.6E-01 | 5.6E-06 |
| NITROTOLUENE, 2- | *SV | S | 137 | 1.9E-01 | 1.2E-05 |
| NITROTOLUENE, 3- | *SV | S | 137 | 2.1E-01 | 2.4E-05 |
| 6PAHs (varies, see Table 4-2b) | *SV | S | – | – | – |
| PHENOL | SV | S | 94 | 3.5E-01 | 3.4E-07 |
| PROPICONAZOLE | SV | L | 342 | 1.0E-06 | 4.1E-09 |
| 7TPH (middle distillates) | *SV | L | 170 | 2 to 26 | 7.2E-04 |
| TRICHLOROBENZENE, 1,2,4- | *SV | S | 181 | 4.6E-01 | 1.4E-03 |
- Physical state of chemical at ambient conditions (V – volatile, SV – Semi-Volatile (*SV – Treated as “volatile” in USEPA risk assessment models if H > 0.00001), S – solid, L – liquid, G – gas).
- Vapor Pressures from National Library of Medicine TOXNET or ChemID databases.
- Check with lab to determine feasibility of wet sieving sample to remove >2mm particles prior to subsampling.
- Soil or sediment samples that consist entirely of <2mm material do not require drying and sieving to address fundamental error concerns, although some degree of drying and sieving may be desirable by the laboratory for testing purposes.
- The stability of a targeted metal depends in part on the species present and can be highly variable. Identification of specific species of a metal may require the collection of aliquots prior to drying and sieving and should be evaluated on a site-by-site basis with respect to the site investigation objectives.
- PAHS – See Table I-3. Eighteen targeted PAHs listed in Section 9 of the HEER Office TGM.
- TPH diesel may not be adequately extractable from soil or sediment when placed in methanol; aliquots should be collected and extracted at the laboratory (e.g., using methylene chloride).
Table I-3 Physiochemical Constants for Targeted PAHs (refer to Appendix K, Section K.6) Reference: Appendix 1, Table H in HEER Office Environmental Hazard Evaluation guidance (HDOH 2017 and updates).
| 3Targeted PAHs | 1PhysicalState | Molecular Weight | 2VaporPressure mm Hg (25C) | Henry’s Law Constant (H) (atm-m³/mol) |
|
| Semi-Volatile PAHs (VP 0.1 to 1.0 OR Liquid at 25C OR Henry’s Constant >1.0E-05) 3,4Subsample Multi Increment Bulk Sample at Laboratory Upon Receipt Without Drying |
|||||
| ACENAPHTHENE | *SV | S | 154 | 2.2E-03 | 1.8E-04 |
| ACENAPHTHYLENE | *SV | S | 152 | 6.7E-03 | 1.5E-03 |
| ANTHRACENE | *SV | S | 178 | 6.6E-06 | 5.6E-05 |
| FLUORENE | *SV | S | 166 | 3.2E-04 | 9.5E-05 |
| METHYLNAPHTHALENE, 1- | *SV | S | 142 | 6.7E-02 | 5.1E-04 |
| METHYLNAPHTHALENE, 2- | *SV | S | 142 | 5.5E-02 | 5.1E-04 |
| NAPHTHALENE | *SV | S | 128 | 8.5E-02 | 4.4E-04 |
| PHENANTHRENE | *SV | S | 178 | 1.2E-04 | 3.9E-05 |
| PYRENE | *SV | S | 202 | 4.5E-06 | 1.2E-05 |
| Non-Volatile PAHs (VP <0.1 AND Solid at 25C AND Henry’s Constant <1.0E-05) 4Dry and Sieve Multi Increment Samples for Preparation of Aliquots |
|||||
| BENZO(a)ANTHRACENE | NV | S | 228 | 5.0E-09 | 1.2E-05 |
| BENZO(a)PYRENE | NV | S | 252 | 5.5E-09 | 4.6E-07 |
| BENZO(b)FLUORANTHENE | NV | S | 252 | 5.0E-07 | 6.6E-07 |
| BENZO(g,h,i)PERYLENE | NV | S | 276 | – | 1.4E-07 |
| BENZO(k)FLUORANTHENE | NV | S | 252 | 9.7E-10 | 5.9E-07 |
| CHRYSENE | NV | S | 228 | 6.2E-09 | 5.1E-06 |
| DIBENZO(a,h)ANTHTRACENE | NV | S | 278 | 9.6E-10 | 1.2E-07 |
| FLUORANTHENE | NV | S | 202 | 9.2E-06 | 8.8E-06 |
| INDENO(1,2,3-cd)PYRENE | NV | S | 276 | 1.2E-10 | 3.4E-07 |
- Physical state of chemical at ambient conditions (V – volatile, SV – Semi-Volatile (*SV – Treated as “volatile” in USEPA risk assessment models if H >0.00001), S – solid, L – liquid, G – gas).
- Vapor Pressures from National Library of Medicine TOXNET or ChemID databases.
- PAHS – Eighteen targeted PAHs listed in Section 9 of the HEER Office TGM. Recommendation to subsample the Multi Increment sample without drying applies primarily to acenaphthene, acenaphthylene, anthracene, fluorene, methylnaphthalenes, naphthalene and phenanthrene and pyrene.
Soil or sediment samples that consist entirely of <2mm material do not require drying and sieving to address fundamental error concerns, although some degree of drying and sieving may be desirable by the laboratory for testing purposes.
APPENDIX J. COLLECTION OF SEDIMENT SAMPLES
Return to the Top of the Page
J.1 GENERAL
Well-thought-out investigation questions and Decision Units (DUs) are required for sediment investigations in the manner as done for soil investigations. Designate vertical DU layers as necessary to meet site investigation objectives, including assessment of risk (human or ecological) and optimization of remediation). Designation of the targeted sediment particle size is an important factor in the selection of tools for sample collection. Consideration of the degree of water saturation is also important, especially at stagnant sites where there is a progressive transition from the water column through a muck layer before reaching semi-consolidated and collectable sediment. Multi Increment (MI) water samples or passive diffusion bag samples might be more appropriate for testing of the muck layer. Refer to Section 3.3.5 and Section 6 for additional guidance and examples.
Multi Increment samples that meet minimum increment number and bulk sample mass requirements discussed in Section 3.6.2 should be adhered to for characterization of designated DUs (see also Appendix D). This includes collection of a minimum 30-increment and 1 to 3 kg sample. Consider water content when addressing the target bulk sample mass. The water content of saturated, fine-grained, clayey sediment can be well over 50%. This might require the collection of a larger sample mass to address sample collection error and provide enough material to the laboratory for testing after drying.
Additional procedural information on sediment sampling is available from many sources including Superfund Program, Representative Sampling Guidance, Volume 5: Water and Sediment (USEPA, 1995b), USGS National Field Manual for the Collection of Water-Quality Data (USGS 2005), and Field Sampling Procedures Manual (NJDEP 2005). These documents focus on past, less-reliable, discrete sample collection methods but still include useful considerations for designation of DUs and characterization of sediment in general.
With proper planning and equipment, the collection of MI samples from sediment in relatively shallow water (e.g., <5 m (5.5 yd) deep) does not involve significantly more effort than required for the collection of surface soil samples. Various types of sampling equipment are available, as reviewed in this Appendix. Consider the type and characteristics of the water body associated with the sediment to be sampled when selecting sampling equipment. Factors include the depth and flow of water, tidal influences, sediment type and consolidation and the thickness/depth of the targeted DU layer(s) of the sediment to be sampled.
If both sediment and surface water samples are collected in the same location, collect the surface water sample first. Refer to HIDOH (2021) for guidance on the designation of DUs and collection of MI-type samples from surface water bodies. If several sediment samples are collected from a streambed, collect the most downstream sample first with subsequent samples collected while proceeding upstream.
Return to the Top of the Page
J.2 SMALL, SHALLOW WATER BODIES
Consider a simple, manual tube-shaped sampler for collection of increments from relatively shallow (<2 m) and easily accessible, calm water. to ensure cylindrical-shaped increments (see Figure J-1). These samplers can be purchased or made by attaching a sturdy, hollow, metallic tube to an adequately long pole. Use of a 2-cm (0.8″) diameter tube will generate an approximately 40- to 50-gram increment for each 10 cm (4″) of sediment, ideal for a 30-increment, 1 to 3 kg sample. A sediment core catcher insert might be needed to collect increments of very loose, sandy soil (Figure J-2).



If the sediment cover is exceptionally thin (e.g., < 10 cm (4 in)) then use of use of a cup-type device or a flat-bottom, scoop sampler might be most practical (Figure J-3). These devices can also be used for the collection of increments from coarser-grained sediment or other situations where use of a core catcher for the tube sampler is not practical. A flat-bottom scoop with upright square sides will also help avoid bias to the upper portion of the sediment (see Figure J-3).
Marking sediment increment collection locations in a stream or canal can be challenging. Consider placing a long, floatable rope (or tape measure) with a marked spacing within the DU (see Figure J-3). A long tape measure with increment positions marked by pins or flags can also be placed along the edge of the waterway to guide sample collection. Refer to Appendix F for guidance on increment spacing in long, narrow DUs.
Take care to minimize disturbance and loss of an increment as the sampling device is being lifted. Contamination is often concentrated in the organic carbon- and/or clay-rich fines fraction. If sediment fines are preferentially lost during increment collection, then the resulting sample will not be representative. Decant excess water from collected sediment MI sample by waiting several minutes and then carefully pouring excess water out of the container. Use a cellulose paper filter to catch and re-place fine sediment back into the container as necessary. Note that the collection of undisturbed, anaerobic sediment samples for geochemical analysis, if required, might require alternative methods. This should be discussed with the overseeing regulatory agency prior to sample collection.
Return to the Top of the Page
J.3 LARGE, SHALLOW WATER BODIES
Alternative tools and methods are required for the collection of sediment samples from larger and/or water bodies up to a few feet deep. Figure J-4 depicts a core sampler used to collect samples from the upper three feet of sediment in an estuary. A clear, 1.5 to 3 foot tube is attached to the end of the extendable push rod. A small boat and Global Positioning System (GPS) device can be used to maneuver to pre-established, increment collection locations within the DU areas.
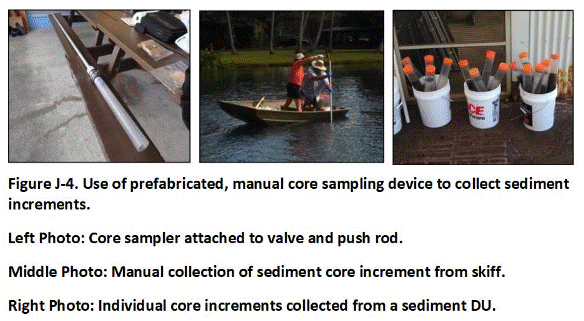
The coring device is manually forced into the sediment to the targeted depth and retrieved. A valve at the top of the sampling tube holds in the sediment as the core is extracted. A cap is fitted to the base of the sampling tube as soon as it emerges from the water to minimize sediment loss. The sampling tube is then removed from the valve/push rod, capped on the other end and stored and the boat is maneuvered to the next increment location.
Small Vibracore tools can also be used for the collection of sediment cores in shallow water (Figure J-5). Very simple, Vibracore-type samplers can be made by attached a vibrating device (e.g., an orbital sander) to a hollow, metal tube of appropriate diameter and length. Use of a Vibracore is described in the following section on the collection of sediment cores in deep water.

Direct-push or other types of sediment core-type samplers can also be mounted to a flat-bottom boat and used to collect sediment samples from water up to 10 m (33′) or more deep. More recent designs include small, weighted platforms that can be lowered to the top of the sediment for coring (Figure J-6; see case study in Appendix C). Core increment collection and sample preparation methods are carried out in a similar manner as described for investigation of subsurface soil in Appendix G. Sediment core samplers have the ability to retain the integrity of sediment horizons with minimal disturbance and allow the collection of unbiased core-shaped increments. Sections of core specific to targeted DU layers, representing increments for those layers, are either removed and combined in their entirety between borings for later subsampling or subsampled in place and a final bulk sample prepared in the field. The latter is identical to subsampling of soil borings described in Appendix G.
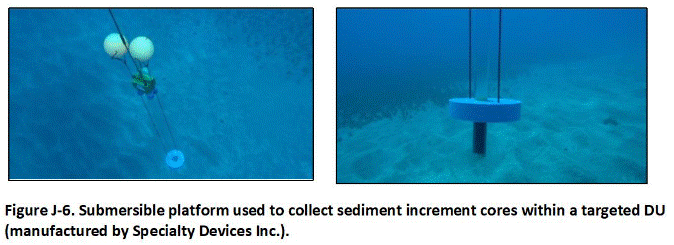
Sample Preparation
Once all cores for a targeted, DU area have been collected, cores are individually extracted and increment sections specific to a targeted, DU layer progressively combined to prepare a sample for that layer (Figure J-7). The base cap is removed from the bottom of the tube and the tube placed on a plunger. The upper cap is removed and the tube is pulled downwards, pushing the core out of the top and progressively exposing individual DU layer increment sections. Once exposed, the increment specific to a targeted DU layer is cut away (e.g., using a stainless-steel spatula) and placed in a container specific to that DU layer (see Figure J-7).
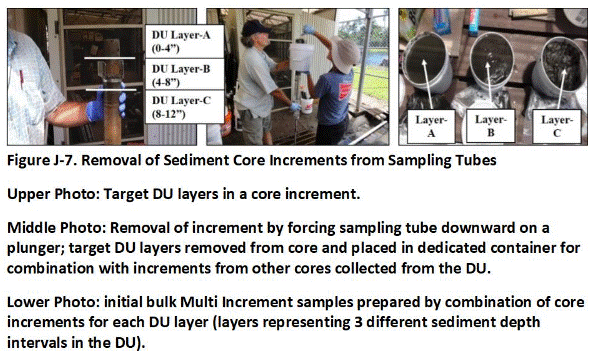

Bulk samples can either be sent to the lab for processing or, if needed due to large sample volume, processed in the field to reduce mass. In the latter case, the sample is placed on clean, plastic sheeting and spread out to a thickness of about 1 to 2 cm (0.4-0.8 in) (Figure J-8). A flat edge spatula is then used to collect subsamples in a systematic, random (grid) fashion. A minimum of 30 subsamples should be collected to ensure that the resulting, reduced sample is reasonably representative of the original sediment collected and minimize error in the final data. Submit the final samples to the laboratory for further processing and testing in accordance with MI sampling methods (refer to Appendix K). The excess sediment for each DU layer can be retained as split samples for additional testing as needed.
Consider the collection of subsamples in the laboratory for testing without drying for sediments that consist primarily of <2 mm particles to reduce sample preparation and analysis time. Drying and sieving are carried out primarily to remove large particles. A sediment moisture content analysis is also necessary if the laboratory subsamples are collected without first drying the bulk MI sample, to report the laboratory data on a dry weight basis.Return to the Top of the Page
J.4 DEEP WATER
Alternative sample collection methods are required for deeper or otherwise less accessible sediment. Vibracore drilling rigs are ideal if available and amenable to the type of sediment to be collected (Figure J-9). Large Vibracore core rigs can be used to collect core increments in water tens of feet deep.

A small pontoon boat equipped with a GPS device can be used to collect increments and prepare samples for submittal to the laboratory final processing and testing. The GPS is used to locate pre-established increment sample collection points. A metal sampling tube with an inner liner is fitted to the base of an electric motor (see Figure J-9). The device is lowered to the sediment interface using a small wench. The motor is then used to vibrate the sampling tube into the sediment. The depth of penetration is monitored at the surface using a tape measure attached to the top of the device. A sediment catch is connected to the base of the tube to retain the sediment core when collected.
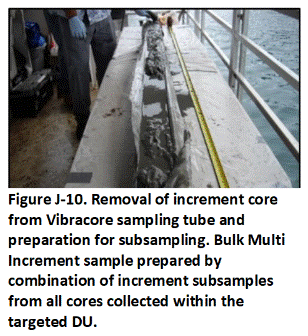
Samples for targeted, DU layers are prepared in the same manner as described above for increments collected in shallow water using more simple devices (refer to Section J.2 and J.3). In this case, however, increment subsamples are collected after a core has been placed horizontally on a table and the full extent of sediment exposed. Identify increments associated with targeted DU layers, considering compaction during collection of the core (Figure J-10). Collect a subsample of sufficient mass from each increment to meet the targeted, bulk sample mass for the DU layer as a whole and place in a container specific to that layer. This might require the removal of multiple, small plugs of sediment from the targeted core length. Avoid the need for additional processing and subsampling in the field to further reduce bulk sample mass.
Increment collection and sample preparation can be expedited by “stacking” multiple increments in a single tube when only a single and relatively thin, surficial DU layer of sediment is to be tested. Assume, for example, that the upper 25 cm (10″) of sediment is designated for sample collection. A 2.5 m (8′) long collection tube can be used to collect 25 cm (10 in) increments from up to eight points without bringing the Vibracore aboard the boat between collection points. After the first increment is collected and held in the tube by the sediment catch, the Vibracore is lifted off the sediment floor one or two meters (3-7′) and slowly moved to the next increment location for collection of another 25 cm (10 in) increment in the same sampling tube. This is repeated at up to six additional increment point locations.
The sampling device is then retrieved to the surface. The core is removed from the sampling tube and the liner cut open to expose the sediment. Determine the target mass of sediment to be removed from the core by dividing the number of increments included by the total number of increments to be collected and multiplying this by the targeted bulk sample mass. For example, if six increments were captured in the core, thirty increments were to be collected and a bulk sample mass of 3 kg to be prepared, then a 600-gram subsample should be collected. This will most efficiently be carried out by collecting regularly spaced, small masses of sediment from the entire length of the core. Smaller masses from a greater number of points will produce the most representative subsample. Refer to Appendix G for the collection of subsamples from subsurface soil cores and Appendix I for the collection of subsamples from cores to be tested for volatile organic compounds for additional guidance.
A potential concern in the use of the Vibracore is the loss of very fine sediment when the sample tube is placed horizontally on the pontoon boat for core liner extraction and the spillage of muddy water from the core. Although the bias introduced into the sample data is likely to be small in most cases, methods to better control this issue include removal of the core from the liner while the tube is in a tilted orientation. This will improve the representativeness of the resulting sample data.
Note that the repeated vibration action of the Vibracore during the period that multiple increments collected in the same sampling tube (as well as the nature of the sediment in that location) can cause the sediment in the tube to disaggregate and run or mix. If sediment collected under these circumstances is to be representatively subsampled, the sediment from the entire core would need to be collected, spread to a thin layer on a large flat surface, and systematic random increments collected, as illustrated in the example in Figure J-8.
Return to the Top of the Page
J.5 DIVER ASSISTED SAMPLE COLLECTION
 Diver-assisted sample collection might also be feasible for the collection of shallow sediment increments and samples in some cases. Sampling tubes of adequate length to penetrate the targeted DU thickness and volume to prepare a 1 to 3 kg sample are carried to the targeted DU area in a netted bag (Figure J-11). The diver manually locates an increment collection point in the same manner as described for the collection of surface soil samples in Appendix F. A sampling tube is then pushed into the sediment to the targeted depth, retrieved, capped on both ends and then placed back into the netted bag. The diver then proceeds to subsequent increment collection points and repeats the process until all increments are collected. When the collection of increments is complete, the diver inflates a buoy attached to the sample collection bag and allows it to rise to the surface, where it is retrieved and brought into the awaiting boat or otherwise taken ashore. Individual increments are then removed from the individual tubes and combined to prepare a final sample.
Diver-assisted sample collection might also be feasible for the collection of shallow sediment increments and samples in some cases. Sampling tubes of adequate length to penetrate the targeted DU thickness and volume to prepare a 1 to 3 kg sample are carried to the targeted DU area in a netted bag (Figure J-11). The diver manually locates an increment collection point in the same manner as described for the collection of surface soil samples in Appendix F. A sampling tube is then pushed into the sediment to the targeted depth, retrieved, capped on both ends and then placed back into the netted bag. The diver then proceeds to subsequent increment collection points and repeats the process until all increments are collected. When the collection of increments is complete, the diver inflates a buoy attached to the sample collection bag and allows it to rise to the surface, where it is retrieved and brought into the awaiting boat or otherwise taken ashore. Individual increments are then removed from the individual tubes and combined to prepare a final sample.
Return to the Top of the Page
J.6 OTHER DEVICES
Other devices used to collect surface sediment samples include center pivot grabs, clamshell pivot grabs and drags, sleds, and scoops (NJDEP 2005; USGS 2005). Use of these types of devices to collect MI samples is problematic due to the large volume of material collected and the need for a large are to combine increments and prepare a final sample for submittal to the laboratory. Loss of fines during retrieval is also a concern. If tested as individual, discrete samples, then the resulting data should be considered adequate for gross, screening purposes in the manner described in Appendix E. This could include approximation of large-scale, contaminant distributions zones that can be used to designate risk- or remediation-based DUs and more carefully tested using DU-MIS sampling procedures.
APPENDIX K: LABORATORY PROCESSING OF MULTI INCREMENT SAMPLES
K.1 INTRODUCTION
There are three primary sources of error in sample data and resulting error in estimates of risk and design of remedial actions: 1) Collection of the sample in the field, 2) Processing and subsampling of the sample at the laboratory for analysis and 3) Analysis of the sample. These errors cannot be completely eliminated, but they can be controlled using proper sample collection, processing and testing methods in accordance with the Theory of Sampling (refer to Appendix D). Collection of an acceptably representative sample weighing only a few kilograms from tens or hundreds of metric tons of soil or other particulate matter in the field is challenging but can be accomplished following the approaches described in previous sections of this guidance (e.g., AAFCO 2015). Error associated with actual analysis of a subsample or analytical “test portion” of a sample is typically lowest and most controllable. This section addresses the second most common source of error in data acquisition and decision making – collection of a representative subsample from a sample submitted to a laboratory for analysis.
Talk to the laboratory ahead of time to ensure that they are familiar with processing and testing of Multi Increment (MI) samples and that their Standard Operating Procedure (SOP) meets sampling theory requirements, as outlined in this guidance document. This includes drying, sieving, and subsampling in accordance with MI collection protocols for non-volatile contaminants. Increments for samples to be tested for volatile contaminants are placed in methanol in the field or upon receipt at the laboratory (see Appendix I). An additional charge for sample processing is normally added to the basic extraction and analysis fee. An additional fee might also be added for testing of a larger subsample mass than required in the analytical method SOP, as discussed below.
Data for samples that are not processed at the laboratory using procedures described in this subsection, or equivalent, cannot reliably be considered representative of the bulk MI provided from the field. Require the laboratory to document specific sample processing and subsample collection methods in the report, rather than simply reference an applicable guidance document. Photographs of the processed samples can also be requested to support the reliability of laboratory data. Include a summary of laboratory processing and subsampling methods in the investigation report.
Bulk samples collected in the field should be kept to a maximum mass of approximately 1 to 3 kg unless otherwise coordinated with the laboratory, due to handling and storage limitations. Larger samples might be necessary in some cases to generate representative samples but should be discussed with laboratory ahead of time. Laboratories might charge extra for processing and disposal of excess material. Sample mass can be reduced in the field using MI subsampling methods if a larger amount of soil is inadvertently collected (see Section F-2 of Appendix F). This is not recommended as a standard practice, however, due to the unavoidable introduction of additional error and uncertainty into the data. Any field processing of bulk samples should be clearly described in the investigation report.
Laboratory processing of MI samples typically consists of the following steps (USEPA 2003b, 2006d; ASTM 2003; AFFCO 2018; ITRC 2020; HIDOH 2021):
- Empty entire bulk sample onto tray made of or lined with material compatible with contaminant of interest and drying temperature;
- Spread evenly into thin layer;
- Allow to air dry until a constant weight is established by re-weighing or air dry until soil agglomerates are crushable;
- Sieve entire sample to the target particle size as defined in the DU designation process (e.g., <2 mm);
- Subsample entire sieved portion using a sectorial splitter (preferred) or manual, MI sampling methods to collect appropriate mass for each targeted analysis (minimum ten grams recommended for the <2 mm particle size for all contaminants; including metals).
Establishment of the target particle size is an important part of the DU designation process and should be discussed as part of the systematic planning process (refer to Section 3.3). Inform the laboratory of the specific particle size range to be isolated and tested for each sample. Separate isolation and testing of multiple particle size ranges might be required in some cases.
Particulate matter <2 mm in diameter is generally considered “soil” for the purposes of an environmental investigation and contaminant analysis, including comparison of data to risk-based action levels (USEPA 2011d). Sieving to <2 mm to remove gravel, sticks and other large debris also establishes the maximum particle size of the sample, which is necessary (in accordance with sampling theory) to determine the minimum subsample mass necessary for extraction and analysis in the laboratory. Note that some agencies or risk assessors might require additional testing of the fines fraction for comparison to screening levels and assessment of risk (e.g., <250 µm or 150 µm). This includes testing for contaminant bioaccessibility.
Sample processing is discussed in more detail in the sections below. Contaminant analyses of all soil samples is normally reported on a dry weight basis. This is in part because soil ingestion rates assumed in human health risk assessments are based on dry weight (USEPA 2011d). Data for samples that are air dried to constant weight and sieved prior to analysis can be considered dry weight without additional analysis for moisture content. Collect a separate subsample test the moisture content in cases where a sample will not be dried to <10% moisture prior to the collection of subsamples for analysis (e.g., Total Petroleum Hydrocarbons as diesel and semi-volatile chemicals). Remaining soil is disposed of by the laboratory, normally after thirty days (consult laboratory for details).
Make arrangements for longer-term storage with the laboratory if archiving of samples is warranted or decisions on potential additional analyses of remaining, processed material might otherwise not be made within the normal, 30-day holding time before samples are disposed of. Do not archive or test individual increments. As discussed in Appendix D, the concentration of a contaminant in an individual increment collected from a Decision Unit (DU) is irrelevant in terms of sampling theory and the objective to obtain the “true” or “mean” for the DU volume of material as a whole. At a small enough scale (e.g., individual particle or coating on particle), the maximum concentration will always be 1,000,000 mg/kg. This fact is immaterial to either risk or the overall objectives of the project.Return to the Top of the Page
K.2 SAMPLE PROCESSING
Samples should be spread into a thin layer (~ 0.5 to 1.0 cm (0.2-0.4 in)) on a large tray and placed in a ventilated area to dry (Figure K-1). This normally takes 24 to 48 hours, depending on the soil type and original moisture condition. Aluminum or plastic trays are commonly used for drying, but should be avoided if aluminum, phthalates or other plastic components are contaminants of potential concern. Paper liners should be avoided if organic carbon is to be tested for or if contaminants are present that could sorb to the paper (e.g., heavy oil).

Samples to be tested for non-volatile chemicals should be air dried under ambient conditions (e.g., 15 to 30°C). Soil moisture content should be reduced to achieve a constant air-dried weight for the samples, as determined by periodic re-weighing or air dry until soil agglomerates are crushable and a separate subsample can be used for moisture analysis and dry weight correction. Drying times can vary between a few hours for course soils with initially low moisture to several days for wet, fine-grained soils. Drying of samples under low temperatures in an oven is acceptable provided that the laboratory has an SOP for this procedure and it can be reasonably assumed that this will not result in significant (e.g., >10%) chemical loss or transformation.
Wet, clayey samples should be periodically crushed with a pestle to avoid formation of hard bricks. Disaggregation should be done in a manner that avoids crushing of rock fragments and other naturally large particles. More intensive particle reduction methods (e.g., grinding) are described below. Be aware that baking a clay-rich sample can result in a brick-like mass that will be difficult to disaggregate and collect a reliable subsample.
Samples should be sieved to <2 mm following drying or alternative, target particle size based on the investigation objectives and then subsampled as described below (USEPA 2011d; see Figure K-1). Note that soil (or sediment) samples that consist entirely of <2 mm material do not require drying and sieving to address fundamental error concerns, although some degree of drying and sieving might be desirable by the laboratory for testing purposes. As noted, data are also normally reported on a dry-weight basis. Exceeding recommended holding times for non-volatile chemicals to permit drying and sieving and minimize subsample collection error is generally acceptable but should be minimized to the extent practicable (USEPA, 2003c). Error associated with poor subsample collection is likely to outweigh error associated with contaminant loss due to exceeding a holding time.
Return to the Top of the Page
K.3 SUBSAMPLE COLLECTION
Of all the laboratory steps necessary to process and analyze environmental samples, subsampling is widely believed to present the greatest potential for error. The lab subsampling guidance applies to all types of soil samples collected in the field, whether MI, discrete, or judgmental samples. The objective from a laboratory standpoint is to ensure that the data generated are representative of the sample provided.
Laboratory error is much easier to control than field sample collection error and should be minimized to the extent possible. Careful subsampling of the processed sample to collect a small mass for extraction and analysis is critical to ensure that the resulting data are representative of the sample submitted as a whole. Refer to AAFCO (2018) for a detailed discussion of the reliability of different laboratory subsample collection methods.
The collection of a representative subsample is most reliably accomplished with a sectorial splitter, also called a rotary riffle splitter (Figure K-2). The availability of a sectorial splitter should be used as one of the criteria for selecting a laboratory. The dried and sieved sample is poured into a hopper at the top of the unit and fed in equal amounts into a series of rotating containers. Note that multiple splits using a sectorial splitter might be necessary to reduce the bulk sample mass down to the desired amount for extraction and analysis.
 Subsamples from milled samples can also be collected using a “two-dimensional Japanese slabcake” methods (Figure K-3). This is also referred to as “fractional shoveling” (AAFCO 2018). The dried and milled sample is spread into a thin (e.g., < 1 cm (0.4″) layer and subsample increments are collected in a systematic random manner, similar to the method used to collect the sample in the field (left photo in Figure K-5). The consistency of finely milled particulate material minimizes the risk that potentially more contaminated, finer-grained particles might settle to the bottom of the slab cake and be underrepresented by a manually collected subsample. A flat-bottomed tool with square sides should, however, be utilized to ensure equal collection of material from the lower and upper portions of the slab and minimize subsample collection error (see Figure K-5). Ensure that the thickness of the slabcake is no greater than the height of the sides of the sampling tool. The final mass of the combined increments should again be sufficient to meet minimum subsample mass requirements for testing, discussed below.
Subsamples from milled samples can also be collected using a “two-dimensional Japanese slabcake” methods (Figure K-3). This is also referred to as “fractional shoveling” (AAFCO 2018). The dried and milled sample is spread into a thin (e.g., < 1 cm (0.4″) layer and subsample increments are collected in a systematic random manner, similar to the method used to collect the sample in the field (left photo in Figure K-5). The consistency of finely milled particulate material minimizes the risk that potentially more contaminated, finer-grained particles might settle to the bottom of the slab cake and be underrepresented by a manually collected subsample. A flat-bottomed tool with square sides should, however, be utilized to ensure equal collection of material from the lower and upper portions of the slab and minimize subsample collection error (see Figure K-5). Ensure that the thickness of the slabcake is no greater than the height of the sides of the sampling tool. The final mass of the combined increments should again be sufficient to meet minimum subsample mass requirements for testing, discussed below.
A sectoral splitter should always be used to collect subsamples of material from dry, un-milled particulate matter that includes particle sizes greater than 250 µm. Subsamples of dry, un-milled samples of particulate material should be collected using a sectoral splitter if at all possible. A 2D slabcake method to manually collect analytical subsamples from dry, un-milled particulate matter should not be used unless the sample has already been sieved to a particle size of 250 µm or less. This is due to inherent separation and settling of potentially more contaminated, finer-grained particles to the bottom of the slab and the unavoidable over collection of particles from the upper portion of the slab using manual subsample collection methods.
In the event that a sectoral splitter is not available, and the sample cannot or otherwise will not be milled, consider collection of subsamples using a “wet,” 2D slabcake method (refer to Figure K-3). This is similar to field subsampling of a sediment sample in the field that is too large to send to the laboratory for processing and testing (refer to Figure J-8 in Appendix J). Use a misting bottle to remoisten the sieved sample to the extent necessary to minimize separation of particles. Manually mix the sample to reduce any distributional heterogeneity of particle sizes caused by sieving. Spread the moistened sample out into a 2D slabcake. Follow the above directions for collection of subsamples in a systematic, random fashion. Consider the collection of additional increments (e.g., 50) and/or subsample mass (e.g., 30 g) as well as replicate subsamples (e.g., 20% of total samples) to further improve data quality.
Cone and quartering methods are never recommended for the collection of laboratory subsamples. Studies have indicated that these methods have a significantly lower subsample data precision in comparison to subsamples collected manually or using a rotary splitter (AAFCO 2018).
Return to the Top of the Page
K.4 ANALYTICAL SUBSAMPLE MASS
Table K-1 summarizes the minimum-recommended subsample mass (analytical sample) for testing based on the subsample collection method and whether the sample was milled. Additional guidance on overall data quality in terms of how the sample is processed and the mass of the subsample collected for analysis is provided in Section K.9.
Subsample masses should be based on Gy’s Theory of Sampling and the need to ensure that the data provided by the laboratory are representative of the sample submitted, not on the minimum mass that can be tested by the laboratory or the desire to minimize costs associated with disposal of waste solvents or use of other laboratory material. The minimum subsample masses noted apply to all contaminants and all analytical methods. A minimum, subsample mass of 10 grams is required to address compositional heterogeneity between individual particles and associated Fundamental Error for samples where the maximum particle size is <2 mm (refer to Appendix D). A larger subsample mass is required for samples with larger particle sizes and must be estimated on a sample-specific basis using equations for Fundamental Error.
Table K-1. The minimum subsample masses noted apply to all contaminants and all analytical methods. A minimum, subsample mass of 5 to 10 grams is required to address compositional heterogeneity between individual particles and associated Fundamental Error for samples where the maximum particle size is <2 mm (refer to Appendix D). A larger subsample mass is required for samples with larger particle sizes and must be estimated on a sample-specific basis using equations for Fundamental Error.
Table K-1. Minimum-recommended subsample (analytical) mass with respect to sample preparation method, maximum particle size and subsample collection method.
| 1Sample Preparation Method | Subsample Collection Method | |
| Sectoral Splitter | Manual | |
| Unground (<2 mm) | 10 g | 30 g |
| Ground (<100 µm) | 5 g | 5 g |
Additional bias and error in the data could be introduced due to lateral and vertical, distributional heterogeneity within a processed sample and associated Grouping and Segregation Error. A minimum, subsample mass of 10 g is considered to be adequate to address both this error and Fundamental Error if a sectoral splitter is used to collect a subsample from an unground sample (Table L-1). This error is more difficult to control if the subsample is manually collected, due to the tendency for fine and coarse particles to segregation during processing. A larger, minimum subsample mass of 30 g is therefore recommended if a manual collection method is used (refer to Table L-1).
Laboratories might charge an added fee for testing larger subsamples than required by current test method Standard Operating Procedures due to the need for additional reagents or other associated costs. This should be considered a necessary, additional cost for obtaining reliably representative data for the sample submitted.
It is possible that some commercial laboratories might not be able to extract or otherwise test a 30-gram subsample. If this is the case, then ensure that triplicate subsamples with a mass of at least 10 grams are collected and tested to assess laboratory subsampling precision (refer to Appendix L, Section L.2).
A smaller subsample mass would in theory be adequate to address Fundamental Error for samples composed entirely of fine-grained particles, for example samples that have been sieved to <250µm. Segregation of coarser and finer particles within this fraction is still unavoidable, however. Physical collection of a representative subsample also becomes problematic for a target mass less than 10 grams, even if a sectoral splitter is used. A minimum 10-gram subsample is therefore recommended for unground, fine-grained samples.
Grinding or “milling” of samples (Section K.4) to <100 µm will significantly reduce concerns for both Fundamental Error and Grouping and Segregation Error as well error associated with the physical collection of a subsample. Milling is not normally carried out on environmental samples as a default but might be desirable or even necessary in some cases. If a case-specific decision is made to mill a sample, then the minimum-recommended subsample mass after milling can be reduced to 5 grams and still address Fundamental Error and subsample collection concerns (Table L-1). Use of a sectoral splitter for the collection of subsamples will in most cases not result in a significant, additional reduction of data error over manual subsampling methods but should be considered if available and feasible.
Physical collection of a representative subsample less than 5 grams again becomes problematic, even if a sectoral splitter is used, and is not recommended even if in theory adequate to address Fundamental Error. If necessary, consider collection of a 30-gram subsample and then use of this mass to collect a smaller subsample. Collect subsample replicates to document subsampling method precision.
If the entire sample cannot be ground due to laboratory limitations, then collect the minimum-recommended subsample mass, use a puck mill to grind the subsample to <100 µm and collect a 5 grams subsample for analysis. Collect and grind replicate, 30-gram subsamples (minimum triplicates) for testing and evaluation of total, subsampling method precision.
Laboratories might need to modify USEPA methods appropriately to achieve the minimum 5- to 30-gram subsample mass for extraction and analysis or conduct multiple small subsample extractions and combine them for analysis. This is primarily a concern for metals, where some methods might call for testing of only 1 gram. With the possible exception of mercury, extraction and testing of 5- to 30-gram subsamples is feasible for most metals if specifically requested. The cost of analysis might increase, but this is the price to obtain reliably representative data and make more confident decisions regarding risk or remediation. Such protocols must be strictly followed to reduce tens or hundreds of tons of soil or sediment down to only a few grams actually tested by the laboratory and generate reasonably representative data for the original DU mass of material as a whole.
Mercury sample extraction mass might be limited to 5 grams or several grams due to the laboratory method involved. If this is the case, then the primary sample should be ground in a manner that does not produce excessive heat and a minimum 5 grams of ground material extracted and tested, with multiple extracts combined and tested as a single extract solution as necessary.
If direct extraction of the minimum-recommended, subsample mass is still not possible due to laboratory limitations, then perform and combine multiple extractions or average data for multiple extractions until the final data are representative of the recommended mass. Collect replicate subsample data to assess data precision. If replicate subsamples result in an RSD of greater than 15%, then consider combining multiple extracts for testing and representation of a larger, total subsample mass.
The latter described steps necessarily introduce additional error into the resulting sample data. Data that do not meet the above recommendations for minimum, subsample mass should be considered suspect. Limitations on data quality and reliability should be noted in the investigation report and incorporated into final decision-making regarding assessment of risk or design of remedial actions.
Return to the Top of the Page
K.5 PARTICLE SIZE REDUCTION
K.5.1 Testing of Un-milled Versus Milled Samples
Testing to Assess Total Contaminant Concentration
Grinding or “milling” of samples beyond crushing of soil clumps by hand or using a simple mortar and pestle can significantly improve extraction of the sample and subsample data precision. Data quality for milled samples can be considered to be consistently “High” to “Very High” if the primary Investigation Question requires estimation of the total (vs bioavailable) concentration of the targeted contaminant (refer to Table K-1). Data quality will improve with increasing subsample mass. Milling of samples can be especially important for design of in situ remedial actions, including the additional of chemicals to soil to reduce the mobility of contaminants. Milling of environmental samples is also specifically required for analysis of some contaminants, for example Method 8330B for explosives residues (USEPA, 2006). This is in part due to the fibrous nature of some residues and the difficulty in obtaining a representative subsample from unground media. Note, however, that leaching tests and are normally run on subsamples from un-milled material collected prior to grinding of the primary samples (e.g., Synthetic Precipitation Leaching Procedure (SPLP) and/or Method 1314 Soil Column methods).
Specific methods for milling of soil and other particulate matter are discussed in Section K.5. Milling is an important part of the mining industry, where an emphasis is placed on estimation of the total concentration of the commodity (e.g., gold) in a targeted DU volume of crushed ore. Rigorous extraction methods are used to separate as much of the commodity from the ore as possible and increase profits. Data quality objectives for marketing of crushed ore typically require the total mass of the commodity present in a stockpile of crushed ore (e.g., iron or gold) to be estimated within a margin of error of less than 5% – far more stringent that normally allowed for collection and testing of environmental samples (refer to Appendix D and Appendix L). The exact error in sample data collected as part of a mining operation is ultimately determined when the commodity of interest is extracted from the processed ore and weighed.
If particle-size reduction of a sample is desired but milling of the entire sample is not possible, then consider use of a sectoral splitter to prepare a minimum 30 g subsample and then mill the subsample. Testing of the entire, milled mass is recommended. If this is not possible due to laboratory extraction limitations, then use a sectoral splitter or a 2D slabcake to collect a appropriate mass from the ground material. A minimum subsample mass of ten grams for milled particles is desirable to ensure high data quality when possible. The collection of reliably representative subsample masses less than 1 g is not physically practicable and should be avoided, even for finely milled material.
Testing to Assess Risk
The need to incorporate milling of samples to be used to assess environmental risk should, however, be evaluated on a case-by-case basis and discussed as part of the site investigation objectives. In contrast to the mining industry, environmental risk is more typically assessed in terms of the leachable or “bioavailable” fraction of the contaminant present in media (refer to Section K.6), rather than the total concentration. Another common investigation objective is to estimate the concentration of the leachable fraction of the contaminant that could be stripped from the soil by infiltrating rainfall and subsequently carried into nearby surface water bodies or to underlying groundwater. Batch leaching tests and soil column leaching tests are normally run on un-milled samples to better reflect true field conditions.
Milling of samples could enhance the extractability of the contaminant in soil and introduce bias into sample data in terms of the risk posed under natural conditions. Risk assessors might prefer to directly test the fines fraction of the soil in the absence of milling rather than grind the entire, <2 mm fraction of the sample. This could overlook contaminants in the coarser fraction of the sample, however. As discussed in Section K.3 and Section K.9, an alternative way to avoid potential bias due to milling and ensure reliable test portions is to collect a larger subsample mass (e.g., 30 g). These data could be combined with bioaccessibility data for the same sample, dependent on the availability of test methods for contaminants of concern (refer to Section K.7).
When available, a sectoral splitter should always be used to collect subsamples from an unmilled sample. Use of a two-dimensional, “Japanese Slab Cake” (2D slabcake) for the manual collection of particulate matter milled to <100µm or for collection of subsamples is acceptable (refer to Section K.3). Use of a 2D slabcake to manual collect a subsample of dry, unmilled particulate matter with particle sizes >100 µm can, however, introduce significant and unrecognized error in sample data (Charles Ramsey, 2023, personal communication; see also AAFCO 2018). This is due to the inherent separation of coarse and potentially more contaminated, finer particles in the slab and the unavoidable over collection of particles from the upper, coarser portion of the slab. Use of a 2D slabcake method to manually collect analytical subsamples from dry, unmilled particulate matter is therefore not recommended under any circumstances. If use of a sectoral splitter is not available and testing of unmilled samples is desired to assess risk, then consider use of “wet-slabcake” subsampling methods described in Section K.5 as an alternative. Refer to Section K.3 for additional guidance on these topics.
If use of a sectoral splitter is not possible, consider rewetting the sample to form a paste and collecting a subsample using a wet-2D slabcake method in a manner similar to that recommended for field subsampling of large, sediment samples described in Appendix J (see Figure J-8). Refer to methods for collection of subsamples from 2D subsamples described above.
Milling of Samples to Address Subsampling Error or Laboratory Method Limitations
Milling of soil and other particulate samples where this was not initially desirable could be required in the following circumstances:
- Presence of large (i.e., > 2 mm) fragments of contaminants in the sample that could contribute to the potential risk to human health and the environment.
- Need to reduce particle size and address subsample collection error identified in replicate subsamples, or
- Need to test smaller subsample masses due to laboratory limitations (e.g., ≤ 10 gram; refer to Section K-4).
Examples of the first scenario include the suspected presence of large chips of lead-based paint in soil around the perimeter of a building. The chips could break down overtime into finer particles. In such cases testing of both un-milled and milled samples should be carried out to evaluate current and potential future risk. The same is true of lead shot in soil. Samples should be milled if particles that could pose potential leaching hazards are present in the sample and could be excluded from the data if un-milled samples are tested (e.g., large nuggets of munitions related compounds such as RDX).
Milling to reduce subsampling error might not be practical in some cases. Releases of PCB containing oils and similar liquids into soil and sediment can lead to the formation of tarry “nuggets,” causing error and highly variable replicate data associated with both samples collection in the field and subsamples collection in the laboratory. Milling of the samples to reduce subsampling error is normally not practical, however, due to smearing of the nuggets on milling equipment. In such cases, the only option to obtain more representative data is to collect and test larger subsamples.
Milling can be especially useful when data for replicate MI samples are highly variable, to help discern if the problem is related to field versus laboratory error. Milling samples to achieve very uniform small particle sizes can help reduce Fundamental Error and improve the precision of laboratory subsampling when replicate data suggest a problem. Milling also allows for a smaller subsample and extraction/analysis mass for non-volatile contaminants.
Milling of a minimum 300 gram of soil is recommended (minimum mass necessary to address Fundamental Error). Milling of larger masses (e.g., 1 kg) is preferable. Milling of a minimum 30-gram subsample is recommended in cases where milling of larger masses is not feasible.
K.5.2 Milling Equipment Puck and ring mills or “puck mills” (Figure K-4) are most commonly employed. Puck mills are able to reach a finer consistency, but can increase the temperature of samples and result in a loss of organic compounds. Puck mills can also normally only grind a small mass of soil at a time. Ball mills are able to mill larger masses of soil (e.g., up to 1+kg), provide more gentle, particle-size reduction and minimize heat generation in comparison to traditional puck mills. Ball mills (Figure K-5) cannot grid a sample to a consistent particle size and should not be relied upon for final sample processing and collection of subsamples. Note that use of an appropriate grinder can add cost to processing and analysis of samples, and might not be available at many labs.
Puck and ring mills or “puck mills” (Figure K-4) are most commonly employed. Puck mills are able to reach a finer consistency, but can increase the temperature of samples and result in a loss of organic compounds. Puck mills can also normally only grind a small mass of soil at a time. Ball mills are able to mill larger masses of soil (e.g., up to 1+kg), provide more gentle, particle-size reduction and minimize heat generation in comparison to traditional puck mills. Ball mills (Figure K-5) cannot grid a sample to a consistent particle size and should not be relied upon for final sample processing and collection of subsamples. Note that use of an appropriate grinder can add cost to processing and analysis of samples, and might not be available at many labs.
 Consider the chemical composition of the mill and target analytes of interest when selecting an appropriate mill. Pucks and rings in puck mills and cylinders in ball mills are typically composed of stainless steel, tungsten carbide or ceramic. Stainless steel pucks and rings or cylinders should, for example, not be used when trivalent chromium is an analyte of interest or when heat generation is a concern (e.g., elemental mercury).
Consider the chemical composition of the mill and target analytes of interest when selecting an appropriate mill. Pucks and rings in puck mills and cylinders in ball mills are typically composed of stainless steel, tungsten carbide or ceramic. Stainless steel pucks and rings or cylinders should, for example, not be used when trivalent chromium is an analyte of interest or when heat generation is a concern (e.g., elemental mercury).
Note that non-elemental, mercury-based compounds used as fungicides at former sugarcane operations such as phenylmercuric acetate are not considered to be significantly volatile or susceptible to loss during processing, especially in aged releases to soil (USNLM 2016). An experienced lab technician can normally control heat generation by milling a sample in short pulses. A ceramic mill can also be used to minimize heating of the sample, if needed. Ceramic equipment can, however, contribute aluminum to the sample.
Return to the Top of the Page
K.6 SEMI-VOLATILE AND UNSTABLE CHEMICALS
Samples to be tested for semi-volatile organic compounds (SVOCs) or non-volatile chemicals with a very short half-life (e.g., <30 days) should be immediately subsampled for testing after receipt by the laboratory and prior to air drying and sieving to minimize significant contaminant loss (e.g., >10% of original mass). Refer to Tables I-2 and I-3 in Appendix I for a list of example compounds.
For the purposes of this guidance, a chemical is considered to be semi-volatile if its vapor pressure is between 0.1- and 1.0-mm Hg or if it is a liquid at 25°C or if the Henry’s Law Constant exceeds 0.00001atm-m3/mol (USEPA, 2019b). Chemicals that fall into this category include Total Petroleum Hydrocarbon as diesel (TPHd), some polynuclear aromatic hydrocarbons (PAHs) and elemental mercury. A chemical is considered unstable if its half-life is less than 30 days. This will most commonly be a potential concern for pesticides with a low persistence. These criteria might be overly conservative for aged chemicals in soil or other factors that could reduce volatility in comparison to fresh product. Discuss the acceptability to subsample without drying and sieving with the laboratory. Note and justify any deviation from the default recommendations in the laboratory report.
Samples to be tested for SVOCs and other unstable chemicals should be immediately cooled after collection. At the laboratory, the samples should be spread out and subsampled prior to drying and sieving. Surface soil samples that have been exposed to air on site prior to sample collection are acceptable for air drying (if needed) even when determining higher vapor pressure SVOCs. This and other alternative approaches should be discussed with the overseeing regulatory agency and described in the investigation Sampling and Analysis Plan. Check with the laboratory to determine feasibility of wet sieving the sample to remove > 2 mm particles prior to subsampling. An effort should otherwise be made to collect < 2 mm particles in lab subsamples (i.e., avoid collection of gravel or larger materials if possible). A separate subsample should also be collected from the wet material in the same manner as done for targeted analytes and used to test for soil moisture, so analytical results can be converted to a dry-weight basis. Ensure that minimum subsample masses discussed in Section K-4 are met or the limitations of the resulting data otherwise noted.
Note that mercury in soils impacted by release of phenylmercuric acetate and similar mercury-based fungicides is not anticipated to be significantly mobile or volatile and normal MI samples processing methods are acceptable (USNLM 2016). When released to soil, these compounds are expected to dissociate forming relatively stable cations and adsorb to organic matter and clay more strongly than the parent compounds. Volatilization from moist soil and water surfaces will not be significant.
Follow standard sample drying and sieving methods described above if additional tests are required for non-volatile chemicals using a different lab analysis. If both semi-volatile and non-volatile PAHs are targeted as contaminants of potential concern, then include testing for both in laboratory subsamples collected from the sample prior to drying and sieving. Note that naphthalene can be reported under most VOC analyses if the laboratory is notified ahead of time.
Return to the Top of the Page
K.7 CONTAMINANT BIOACCESSIBILITY
Adjustment of sample data to reflect the predicted, bioaccessibility and bioavailability of the targeted contaminant is acceptable if the test method has been approved by the overseeing, regulatory agency, Risk-based screening levels developed for soil normally assume that 100% of the target contaminant will be released in the receptor’s digestive tract and available for uptake into the body.
Only a fraction of the contaminant is likely to be stripped from the soil and enter the blood stream. The remainder is excreted along with the soil particles. The portion of the contaminant that stripped from the soil and enters the gut (or lung fluids) is referred to as the “bioaccessible” fraction. The portion of the bioaccessible fraction that actually enters the blood stream and cells, where it could cause harm, is referred to as the “bioavailable” fraction.
Expensive and time-consuming animal testing is usually required to accurately estimate the bioavailable fraction of a contaminant in soil. As an alternative, relatively simple methods that estimate the bioaccessible fraction have been developed for some chemicals. In short, the total concentration of the contaminant in the soil sample is first determined through normal analytical methods. A second subsample of the soil is then collected. The mass of the contaminant in the subsample is estimated based on the initial concentration data. The subsample is then placed in a solution of artificial, digestion fluid and agitated for many hours. The fluid is then tested and the concentration and mass of the contaminant that moved into the fluid estimated.
The ratio of the fraction of the contaminant that was stripped from the soil to the original mass of the contaminant in the soil represents the bioaccessible fraction. The bioaccessible fraction is conservatively assumed to represent the bioavailable fraction of the contaminant, even though some of the contaminant stripped from the soil is likely to pass through the digestive tract without being taken up into the body.
The total concentration of the contaminant initially reported for the sample is multiplied by the bioaccessible fraction and the bioaccessible concentration of the contaminant calculated for comparison to risk-based screening levels. Data for related samples are similarly adjusted by the same, bioaccessible fraction for comparison to screening levels.
Consult with the local regulatory agency, laboratories and other experts for methods to test the bioaccessibility of other contaminants in soil. Bioaccessibility test methods are well developed for lead and arsenic Ruby et al. 1996; SBRC 1999; Ruby 2001; Kelly 2002; Juhasz 2007; HIDOH 2023). Methods are also being developed for other metals and contaminants. The test methods normally recommend that the <250 µm or finer fraction of the soil be tested. The entire, original sample should be sieved to the target, particle size fraction without grinding. Multi Increment subsampling methods should then be used to collect subsamples for total contaminant concentration and bioaccessibility testing.
Return to the Top of the Page
K.8 SOIL LEACHING TESTS
Processing and testing of soil samples to ne sed to assess potential leaching concerns requires additional considerations. Natural soil leaching concerns can be assessed by use of Synthetic Precipitation Leaching Procedure (SPLP, USEPA Method 1312), soil column leaching method (Method 1314) or, for unpaved areas and aged releases, simple testing of groundwater (HDOH 2024). Leaching risks for soil that is to be disposed of in a landfill is assessed based on the Toxic Characteristic Leaching Procedure (TCLP, USEPA Method 1311).
Both the SPLP lab protocol (USEPA 1994c) and TCLP lab protocol (USEPA 1992g) require testing of a minimum 100-gram subsample. Large particles included in the sample submitted to the laboratory should be included in the leaching test. This is because the Investigation Question relates to leaching of contaminants from the soil as a whole. Particles over 0.5 cm (0.2 in) in diameter should be manually broken into smaller pieces in order to meet a Fundamental Error of approximately 15% (refer to Appendix D). Note that the USEPA lab methods only require breakage of particles to only 1 cm (0.4″) diameter or less. This would result in an unacceptably large Fundament Error, however. Samples should not be milled, since this could bias the leaching tests high.
Return to the Top of the Page
K.9 ASSESSMENT OF OVERALL SUBSAMPLE DATA QUALITY
The method used to collect a subsample will directly control the reliability and quality of the resulting analytical data as well as decisions made based on the data. Subsample mass and particle size uniformity are two important controlling factors. It is also important to refer to the Investigation Question(s) that formed the basis of the investigation for which the sample was collected. Key questions in particular are key to most investigations in order to address risk and/or to optimize remediation:
- “What is the concentration of bioavailable contaminant in the targeted Decision Unit (DU) volume of soil (or other targeted media)?”; and
- What is the total concentration of the contaminant in the Decision Unit volume of soil (or other targeted media)?”
The answers to these two questions are not necessarily identical. Grinding a sample to a consistent particle size (e.g., < 100µm) using a puck mill will significantly reduce error in collection of a representative subsample (refer to Section K.5). Milling will also allow collection and testing of a smaller subsample mass and increase extraction efficiency of the contaminant from the media, due to the increase in surface area over mass. In combination, these factors will significantly decrease laboratory error with respect to Question 3 above.
Contaminants such as arsenic and highly sorptive organic compounds are often tightly bound to soil particles, however, and not bioavailable. Such contaminants are not part of the DU in terms of assessment of risk and Question 1 above. The same milling process that decreases sample data error in terms subsample collection and extraction efficiency can increase sample data error in terms of the estimated concentration of bioavailable contaminant in the sample. The reported concentration of the contaminant in a milled sample can also lead to error in estimation of the concentration of leachable contaminant from the soil for the same reason (Question 2). The resulting data in these cases should not be thought of as more “conservative,” potential error in the data and in final decision making on actions to be taken in the field is simply greater.
Balancing the need to ensure collection of a representative subsample while adhering to the needs of the Investigation Question(s) must be evaluated on a site-specific basis. Varying methods of subsample collection will generate varying degrees of data quality and reliability for these questions, as summarized in Figures 6 and 7.
Data quality for 30 g subsamples collected from un-milled samples collected using a sectoral splitter is considered “Very High” for initial assessment of risk. Further testing of subsamples for bioaccessibility or bioavailability using methods specifically designed for these parameters will improve data quality and decision making (refer to Section K.7). Data quality for milled samples can be considered to be consistently “High” to “Very High” for estimation of total contaminant concentration when required as part of the investigation objectives. Data quality in both cases will improve with increasing subsample mass.
When available, a sectoral splitter should always be used to collect subsamples from an un-milled sample. Use of a two-dimensional, “Japanese Slab Cake” (2D slabcake) for the manual collection of particulate matter milled to <100µm or for collection of subsamples is acceptable (refer to Section K.3). Use of a 2D slabcake method to manually collect analytical subsamples from dry, un-milled particulate matter is not recommended under any circumstances. This is due to inherent separation and settling of potentially more contaminated, finer particles at the bottom of the slab and the unavoidable over collection of particles from the upper portion of the slab using manual subsample collection methods. Alternative, although somewhat less reliable subsample collection methods, are discussed in Section K-3.
APPENDIX L. COLLECTION AND EVALUATION OF REPLICATE SAMPLE DATA
Return to the Top of the Page
L.1 COLLECTION OF FIELD SAMPLE REPLICATES
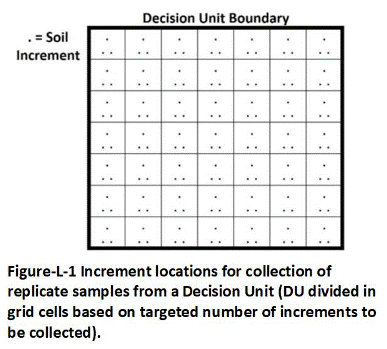 Three independent field Multi Increment (MI) samples (primary plus two replicates) should be collected and tested from at least 10% of the Decision Units (DUs) designated as part of a site investigation to assess the precision of the overall sample collection and analysis method. This is accomplished by collection of three increments from each increment grid cell designated for the DU (Figure L-1).
Three independent field Multi Increment (MI) samples (primary plus two replicates) should be collected and tested from at least 10% of the Decision Units (DUs) designated as part of a site investigation to assess the precision of the overall sample collection and analysis method. This is accomplished by collection of three increments from each increment grid cell designated for the DU (Figure L-1).
If a single sample is to be collected from the DU, then a single increment is collected from the center of each grid cell and then combined to prepare a single, MI sample (refer to Section 3.6.2). Three increments are independently collected from each grid cell for preparation of triplicate samples, one increment for each sample. Label the replicate samples “A”, “B” and “C” at the end of the DU identification code (e.g., DU-1A, DU-1B, DU-1C).
Place an equilateral triangle over the center point of the grid (Figure L-2; real or imagined). Label each point “A”, “B” and “C.” Each point represents the increment collection location for one of the three replicate samples. Collect an increment from each point in separate containers labeled with the sample identification number (e.g., plastic bucket with sample identification number taped to side).
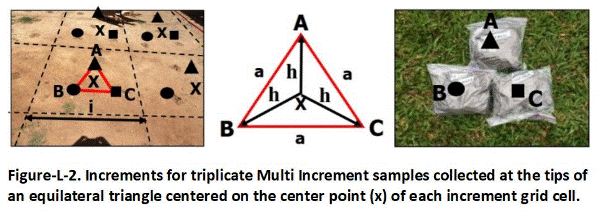
Use a triangle side length (a) equal to 1/3rd of DU increment spacing (i). This will provide adequate spacing between replicate sample increments. The length “h” from the equilateral center point to the increment sample collection points at the tips of the triangle is calculated by the formula:
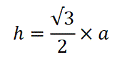
Assuming the side of the triangle “a” equal to 1/3rd of the increment spacing “I” yields:
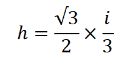
or (rounding to one significant digit):
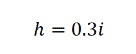
An appropriate distance from the center of each grid cell for the collection of increments for triplicate samples is therefore 30% of the increment spacing determined for the DU as a whole. The increment for the first sample is collected immediately above the grid cell cent point (Sample A in Figure L-2). An increment for each of the two duplicate samples is then collected below and 120 degrees to the left and to the right of the center point (Sample B and Sample C in Figure L-2). Laboratory subsample replicates are collected in a similar manner as described above for field samples. Subsamples should be collected in an identical manner and meet minimum requirements for total number of increments and subsample mass.
Return to the Top of the Page
L.2 COLLECTION OF LABORATORY SUBSAMPLE REPLICATES
The collection of laboratory subsamples is described in Appendix K. Subsample replicates are collected either using sectorial splitter or manually in much the same way as replicate samples are collected in the field. If collected manually, ensure that subsample increments are collected in a systematic, random manner from independent locations with the sample. Be careful to collect the entire mass of soil or other particulate media at the increment location, including fines that might have settled to the bottom of the spread-out sample.
Replicates collected from methanol-preserved samples are used to assess analytical precision, rather than the precision of the method used to collect an aliquot of methanol from the sample container (refer to Appendix I). The potential for volatile contaminants to be unevenly distributed within the methanol and server as a source of data error should be discussed with the laboratory if a significant variability in subsample data is reported.
Return to the Top of the Page
L.3 EVALUATION OF DATA USABILITY
L.3.1 Review of Field Sample and Laboratory Subsample Collection Methods
The evaluation of data quality begins with a review of the method used to collect samples in the field and subsamples in the laboratory. Refer to Section 3.8 for a checklist of sample and subsample collection methods.
If the field sample and/or laboratory subsample was not properly collected, as described in this guidance, then the reliability and representativeness of the resulting data must be considered unknown even if the precision of replicate data is very good. This is because statistical tests only assess the ability of the test utilized to estimate a mean for the data set provided. Statistical tests do not directly assess the actual representativeness of the data set provided.
L.3.2 Calculation of Replicate Sample RSDs
The total precision of MI sample data is evaluated based on a comparison of data for replicate samples collected from the same DU. Replicate sample data and data usability are specific to individual contaminants. Replicate data might vary significantly for some contaminants identified in a DU and only slightly for others.
Acceptance criteria for the statistical evaluation of the sample data are established as part of the Data Quality Objectives process for the site investigation. A two-step process is presented. The Relative Standard Deviation (RSD) of the contaminant concentration reported for each replicate sample is first calculated. This provides a measure of the precision of the MI sampling method used to estimate the mean contaminant concentration for the DU in terms of combined field and laboratory error.
Data precision is evaluated by comparing data for replicate samples collected from the same DU. Replicate MI samples are intended to provide estimates of the mean concentration of a contaminant in a DU that approximate a statistically normal distribution. This allows statistical evaluation of data with as few as three replicate samples. The precision of the data for a given DU can be evaluated in terms of the Standard Deviation (SD) or more specifically the Relative Standard Deviation (RSD) of replicates. The SD and RSD reflect the total sum of field and laboratory error in the data (i.e., field sampling error + lab processing/subsampling error + lab analysis error).
The RSD represents the ratio of the standard deviation of the replicate set over the mean of the replicate set, expressed as a percentage:
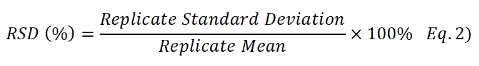
The lower the RSD the more precise the sampling approach used, and the more reproducible the data. Replicate MI sample data for the same DU should be normally distributed if the samples are properly collected (Pitard 2019; Esbensen 2020). An RSD <35% confirms potential normal distribution of the data and is considered to reflect good precision for estimates of the mean. This implies that the sampling method used, including the number, spacing, and size/shape of increments and total mass of soil collected was adequate to capture and reflect small-scale heterogeneity of contaminant distribution within the DU and that error in the laboratory processing and analysis methods was low.
As discussed below, an RSD of 35% is considered to indicate good reproducibility and reliable data for decision making. An RSD of >100% is considered to be very poor, and not typically appropriate for final decision making (see discussion below). An RSD of <15% is desirable for laboratory subsample replicate data, although a higher RSD might be required for analytical methods with an inherent poor precision. High RSDs otherwise suggest poor subsampling methods and/or an inadequate subsample mass. Use of a sectoral splitter or even milling (grinding) of the sample might be required to achieve acceptable replicate data results for samples that contain small chips or nuggets of contaminants.
A low standard deviation for soil sample data is achieved by minimizing error in sample collection, processing and analysis to the extent feasible. This, in combination with a low standard deviation of replicate data, indicate high confidence in the representativeness of the data for decision making. When the mean concentration of a contaminant reported for a set of MIS replicate samples is close to the screening level, a lower standard deviation for the replicates provides stronger evidence that the true DU mean is indeed below the action level.
Confidence in the representativeness of data for a single MI sample decreases as the precision of the replicate sample data decreases. An RSD (e.g., >35%) calls into question the normal distribution of the replicate sample data and could be associated with error in the field and/or laboratory. Field sampling error is the most likely source of data variability. Inadequate sample processing and subsampling is the main source of error at the laboratory, rather than analytical error. This can be evaluated by a review of sample collection, processing and subsampling procedures, as well as testing of replicate samples. The field replicate RSDs are used to estimate the total error for the sample data. The lab subsampling and analysis RSDs are used to estimate the lab subsampling and analysis error for the sample data.
The laboratory subsampling and analysis error can then be subtracted from the total error to compare errors attributable to 1) Field sampling, and 2) Laboratory subsampling and analysis. This analysis should be routinely carried out to evaluate sample data and help identify errors that might be corrected. In limited instances, grinding of samples in the laboratory might be required to reduce the grain size and allow the collection of more representative subsamples, since the ability to increase the mass of soil extracted and tested is limited (see Appendix K).
If the RSD for field replicate samples (total error) is unexpectedly high and RSD(s) for the lab subsampling and analysis replicates are reasonably low, then collection of the samples in the field is the likely source of error. A high RSD typically indicates either an inadequate number of increments used to prepare the samples and/or the presence of small nuggets of the contaminant in soil. The precision of field replicate samples for a DU can be improved by increasing the number of increments and total sample mass to provide better coverage and sample support.
Note that the replicate data only evaluate the precision of the overall sample collection and testing method; i.e., the reproducibility of the sample data. The accuracy of the data with respect to the true mean concentration of the contaminant in the subject DU area and volume of soil can only be known by extracting the chemical from the entire volume of soil and measuring the mass. This is routinely done in mining operations (e.g., extraction of gold from crushed ore) but not as part of most environmental investigation and remediation projects, although error in sample data can sometimes be estimated as part of an in-situ remediation project. The total error in the data therefore also cannot be determined. The only conclusion that can be stated is that the samples were collected in accordance with Gy’s sampling theory and that the precision of the data is good, moderate, poor or very poor.
L.3.3 Data Usability Based on Replicate Sample Precision
Table L-1 presents a recommended approach for evaluation of DU data based on a review of replicate sample data, either collected directly from the DU in question or based from replicate data from similar DUs. Although somewhat subjective, the approach helps minimize the need to re-sample DUs when proper field and laboratory protocols are followed, while balancing the need to ensure that significant risks to human health and the environment are not inadvertently missed.
If multiple sets of replicate samples were collected from a similar, targeted area, then refer to the replicate data with the highest RSD to assess overall data precision. If separate sets of replicate samples were collected from anticipated low-concentration and high-concentration areas (recommended), then use the RSD calculated for each data set to separately evaluate the precision of data for the respective areas.
RSD ≤ 35% (Good Data Precision)
Direct comparison of unadjusted DU data, or the arithmetic mean of replicate data to target action levels, is acceptable when the RSD of the representative replicate data set for the contaminant of concern is less than 35%. This assumes, of course, that the samples were collected, processed, and tested in an unbiased manner and are reasonably representative of the targeted DU. If soil remediation is carried out then unadjusted DU data can be used for confirmation samples.
35% < RSD ≤ 50% (Moderate Data Precision)
An RSD ≤ 35% but > 50% indicates less reliable but in most cases still acceptable for decision making. A thorough review of field and laboratory procedures should be included in the site investigation report. This review can help identify the need for improvements in field or laboratory methods for future investigations.
Error associated with the sample data can in most cases be assumed to be well within the margin of safety incorporated into most risk-based screening levels. Exceeding a screening level does not indicate that adverse health effects will occur. Toxicity factors and exposure assumptions used to establish safe levels of exposure and develop screening levels typically include a minimum ten-fold safety factor (USEPA 2002j, 2005h).
The collection of additional MI samples is recommended for confirmation of remediation of DUs that exceeded action levels, even if Boundary DU data collected during the initial investigation were below screening levels. The confirmation sampling should include the use of a greater number of increments per DU and sample mass and/or division of the area into smaller DUs for re-characterization.
50% < RSD ≤ 100% (Poor Data Precision)
If the RSD of the replicate sample data is between 50% and 100%, it is necessary (again) to review the on-site sampling method and laboratory processing and analysis methods in the investigation, and to discuss the potential causes of the error. Review laboratory replicate sample data to determine if the error might be associated with sample processing and testing, rather than collection in the field.
If laboratory error is suspected, then one or more of the following methods should be used to improve subsampling precision (refer to Appendix K): 1) Use a sectoral splitter to prepare laboratory subsamples rather than manual subsample collection; 2) If manual collection of subsamples is still required, then increase the number of increments used to prepare the subsample; 3) Increase the mass of the laboratory subsample to 30 grams for unground samples or 4) Grind each sample and collect a minimum 10-gram subsample using a sectoral splitter. If data quality adequately improves, then the same method should be used to process and test all other samples.
If field error is determined to be the cause of the problem, then there are two options: 1) Collect new samples from the affected DUs with a larger number of increments and greater total mass or 2) Use the maximum-reported concentration of the contaminant for DUs with replicate sample data and adjust data for all related DUs upward by the replicate DU RSD. For the first option, increase collect a minimum of 75-increment samples and ensure a minimum, 2 to 3 kg total sample mass. The collection of more than 100 increments per sample is usually not considered beneficial. If replicate precision is still poor in this scenario and the need for remediation still uncertain, then consider the collection of a larger mass of soil and subdivision of the DU into smaller areas for separate testing.
Under the second option, adjust data for a DU where replicate sample data were not collected upwards by the RSD calculated for the replicate sample data set associated with that DU. This approach treats the reported concentration of a contaminant as a hypothetical, replicate sample set mean and generates the highest concentration of the contaminant that would be reported if replicate samples were collected and the same RSD applied. Use of the sample data in this manner should be carried in in coordination with a risk assessor trained in DU-MIS sampling methods and Gy’s Theory of Sampling.
Note that the collection of additional samples is not necessary when the reported concentration of the contaminant is well above the screening or cleanup level and there is high confidence that the DU requires remediation. One exception might be the need to obtain a more accurate estimate of total contaminant mass for heavily contaminated DUs to assist in the design of in situ or ex situ remediation projects.
RSD > 100% (Very Poor Data Precision)
If replicate sample data exceed an RSD of 100%, then both the highest concentration reported for a set of replicate samples and the adjusted concentration of sample data for DUs where replicate samples were not collected cannot be considered to be conservatively representative of the true mean. Very high RSDs can be related to the presence of nuggets or chips of the contaminant in the soil. High RSDs can also be generated as the reported concentration of the contaminant approaches the laboratory reporting level.
Retesting is not required for DUs where the need for remediation is already clear from the data and other field evidence but retesting of other DUs should be considered. A review of field sampling methods and laboratory processing and testing methods should again be evaluated and potential sources of error in the data determined. If laboratory error is suspected, then follow guidance presented above for retesting of samples and re-evaluate corresponding replicate sample data.
Addressing error in the field requires the preparation of larger mass samples from a larger number of increments and possible subdivision of initially tested DUs to better isolate potentially high-concentration areas (refer to Appendices F–J). Addressing laboratory error similarly requires testing of larger mass subsamples from a larger number of points and/or grinding of samples (refer to Appendix L).
In cases where DU data are substantially lower than target screening levels, potential use of the data in a similar manner as described for cases where the replicate RSD falls between 50% and 100% should be reviewed in coordination with a risk assessor trained in MI sampling methods and Gy’s Theory of Sampling.
Larger-mass samples composed of a greater number of increments should be collected to confirm contaminant concentrations in high-risk exposure areas and to confirm any remedial actions.
Localized areas of heavy contamination within a DU can result in an elevated RSD for replicate samples if the samples are not properly collected (i.e., adequate number of increments and bulk sample mass). If known or suspected to be present, then such “source areas” should be designated as a separate DU and independently characterized at the beginning of the project (refer to Section 3.3.2 and Appendix C). This will also assist in optimization of remedial actions, if required.
Belated dividing of an initial Exposure Area DU into smaller DUs for characterization might or might not be beneficial, depending on the nature of contaminant distribution. The use of smaller DUs might not improve data precision, if the contaminant is evenly dispersed throughout the DU but highly heterogeneous at the scale of an individual increment. In this case, an increase in the number of increments collected and the mass of the sample collected will be necessary to obtain representative and reproducible data.
Table L-1. Recommendations for assessment of data quality based on the relative standard deviation of replicate samples.
| Replicate Sample Data Precision | Use of DU Data for Decision Making |
| Good (RSD≤35%) |
|
| Moderate (35%<RSD≤50%) |
|
| Poor (50%<RSD≤100%) |
|
| Very Poor (RSD>100%) |
|
APPENDIX M: GENERAL FIELD OPERATIONS
Return to the Top of the Page
M.1 FIELD DOCUMENTATION
M.1.1 Surface Soil Sample Logs
Accurate field logs are essential for evaluation and interpretation of analytical results and for preparation of the site investigation report. Details of all field activities, both during initial site inspections and during sample collection, should be recorded. The latter includes documentation of the possession and handling of samples from the time of collection through analysis and final disposition. Copies of the sampling logs, pertinent notes, and photograph logs should be included in the site investigation report.
Similar information should be recorded for borings, trenches, or pits installed to collect MI samples from subsurface DUs as well as the use of any discrete soil samples for initial screening purposes. Deviations from the sample plan caused by conditions encountered in the field (e.g. inadequate tools for sample collection, unanticipated surface debris or subsurface obstacles in sample collection, weather delays, etc.) are also important to document. Site visits to assess field conditions prior to mobilization for the collection of samples are essential and should also be documented in field logs.
An example of a surface soil sampling log is presented as Figure M-1. Include similar information for any discrete soil samples collected. The intended use of the discrete samples and limitations regarding their representativeness should be clearly discussed in the investigation report (refer to Appendix E). Copies of the field logbook, sampling logs, and (if available) photograph logs should be included in the site investigation report.A log should be prepared for each DU included in the investigation. The logs should at a minimum include the following information:
 Adequate information should be included in the sketch map and sample information log to generate a to-scale map of DU locations in the final report (e.g., shape, dimensions, adjoining DUs, landmarks, north arrow, etc.). Depictions of DUs on high-altitude (e.g., satellite) maps or with low-altitude (e.g., drones) photos is acceptable and even preferred provided that distortion is not too great to prevent accurate estimation of dimensions (Figure M-2). Draft DU maps can be made prior to sample collection and adjusted in the field as needed. Final DU maps depicted on aerial imagery can significantly aid in future re-identification of investigated areas.
Adequate information should be included in the sketch map and sample information log to generate a to-scale map of DU locations in the final report (e.g., shape, dimensions, adjoining DUs, landmarks, north arrow, etc.). Depictions of DUs on high-altitude (e.g., satellite) maps or with low-altitude (e.g., drones) photos is acceptable and even preferred provided that distortion is not too great to prevent accurate estimation of dimensions (Figure M-2). Draft DU maps can be made prior to sample collection and adjusted in the field as needed. Final DU maps depicted on aerial imagery can significantly aid in future re-identification of investigated areas.
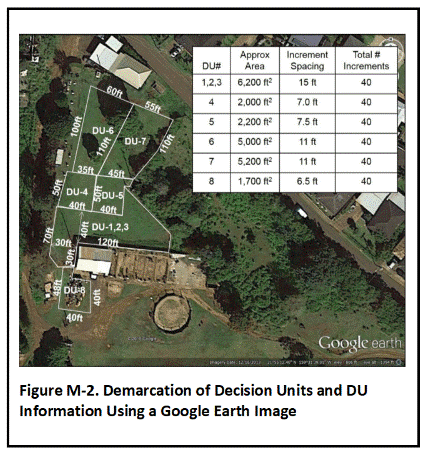 Coordinates determined via hand-held Geographic Positioning System (GPS) equipment is acceptable to record the boundaries of Decision Units (e.g. four corners of a rectangular-shaped Decision Unit). The accuracy of the equipment to be used should be documented in the SAP. Any potential variability caused by surrounding forests, structures, or other obstructions to the GPS unit acquiring satellite signals should be taken into account and documented in the investigation report.
Coordinates determined via hand-held Geographic Positioning System (GPS) equipment is acceptable to record the boundaries of Decision Units (e.g. four corners of a rectangular-shaped Decision Unit). The accuracy of the equipment to be used should be documented in the SAP. Any potential variability caused by surrounding forests, structures, or other obstructions to the GPS unit acquiring satellite signals should be taken into account and documented in the investigation report.
A more detailed survey of the site and DU boundaries by a surveyor licensed in the State of Hawai‘i is required for maps to be included in an Environmental Hazard Management Plans (EHMP). This is necessary to more precisely document locations where contamination will be left in place for long-term management. The EHMP should include the latitude and longitude of key DU boundary points, along with ground surface elevation data determined within the Hawai‘i State Plane Coordinate System to an accuracy of 0.1 foot. Locations of existing buildings or other major landmarks should also be surveyed for reference to the targeted area under long-term management.
The basic rational for designation of the DU (e.g., suspect spill area, perimeter DU, etc.; see Section 3.3) should be noted in the site documentation. The tools and method used to collect Multi Increment (or other) samples should be described in the sample logs. Note the increment spacing used for each DU (see Section 3.6.2). Record any field processing of the sample(s) collected, including the need to reduce the sample mass due to the original bulk sample(s) being too large for lab processing. Field processing of surface soil samples beyond the removal of large rocks and debris and sieving, if the soil is adequately dry and clay-free, should be avoided (refer to Appendix K).
Record information for field observations and field screening methods used to assist in the identification of potential contaminants of concern or test samples in the field (see Section 8). This might include visual and olfactory observations, or the use of tools such as a photo-ionization detector (PID), portable X-ray fluorescence device (XRF), immunoassay test kits or a field Gas Chromatography-Mass Spectrometry [GC/MS] unit. A dehumidification tip should be used with the PID. Field measurements should be recorded and included in the log for the targeted DU.
Recording the spacing of increments is important in order to document that the sample was collected in a systematic random manner. As discussed in Section 3.6.2, the specific locations of individual increments do not need to be recorded or included on a map of the DU. This is in part because individual increments cannot be assumed to be representative of the immediately surrounding soil, due to uncertainties from random small-scale variability (refer to Appendix D). The exact location of any given individual increment collection point therefore does not need to be documented.
A basic soil description and classification should be included with the sample log (see Appendix M). Additional and more detailed information regarding soil taxonomy, geotechnical properties and other characteristics that might be required to meet the project objectives should be included as appropriate. A brief overview of consistency, cementation, structure, rock classification, and other information is provided in Appendix M.
M.1.2 Subsurface Soil Sampling/Boring Logs
Logs for samples collected from subsurface DUs should include the same type of information noted above for surface soil samples regarding site conditions, sample collection methods, boring spacing, etc. Record the rational for the selection of targeted DU layers in the same manner as done for surface soil (refer to Section 3.3). This might include the anticipated known or anticipated depth of contamination, visual or olfactory evidence of contamination during test borings or abrupt changes in soil characteristics. Field processing of subsurface Multi Increment samples is typically required due to the mass of individual core increments and should be described in the logs (refer to Appendix G).
Additional information is required for exploratory borings or borings to be used for the installation of wells. A log for each boring should be prepared which includes the following items, as applicable:
 An example boring log is depicted in Figure M-3. The soil descriptions should also include information on density or consistency (primarily when borings are conducted using hollow stem auger) and appearance descriptors of cementation, structural appearance of layers and other features, and other appearance descriptors as applicable to the project.
An example boring log is depicted in Figure M-3. The soil descriptions should also include information on density or consistency (primarily when borings are conducted using hollow stem auger) and appearance descriptors of cementation, structural appearance of layers and other features, and other appearance descriptors as applicable to the project.
M.1.3 Soil Description
Soil descriptions for soil sampling events should be made by a trained geologist, geotechnical engineer or soil scientist. The Unified Soil Classification System (USCS) is recommended for basic soil descriptions (ASTM 2006d; see also ASTM 2006e; USDA 1987, USDA-NRCS 2007; Nielsen 2006; US Navy 2007).
The level of detail appropriate for soil descriptions for a given site is tied to and should be addressed in the site investigation objectives (DQOs). At a minimum the color and estimated nature of the soil in terms of average particle sizes (e.g., gravel versus sand versus clay) should be recorded using a standardized approach.
More detailed soil descriptions, including laboratory-based measurement of particle size distribution, might be required for more detailed investigations of contaminant fate and transport or to design remedial actions. Maps with taxonomic names for soil on each island are published by the US Department of Agriculture (USDA, 2015). The accuracy of the maps in highly developed, urban area where soil from other areas may have been imported should be verified.
A summary of key elements of the classification system is provided below. If an alternative classification scheme is used then a summary of terms used and particle size categories should be included in the report.
Recommended Parameters for Soil Descriptions
Classification of soil in accordance with the USCS involves a group symbol, name, and complete word description (ASTM, 2006d). Key descriptive parameters include:
- Relative proportion of gravel, sand and fines and USCS classification name and symbol;
- Color (Munsell method preferred);
- Consistency;
- Moisture content;
- Staining/discoloration/odor;
- Glass, wire, porcelain fragments or other debris indicative of disposal or fill;
- Other descriptive terms
Descriptive terms denoting the geologic nature of the soil can also be added, including such terms as “saprolite” for soil directly derived from weathered rock or “sediment” for soil associated with unconsolidated terrestrial or marine sediments. Additional soil descriptors can be included as needed based on the DQOs of the investigation (e.g., plasticity, angularity, etc.; refer to ASTM, 2006d). Properties regarding the in situ structural nature of the soil might be required in projects that include a geotechnical component (e.g., density, structure, etc.). A review of properties commonly recorded as part of subsurface borings is provided in Appendix M.
Include notes regarding odors and other observations during drilling, even if these are not apparent in the samples collected. Field aids that combine the USCS classification system with examples of particle sizes, percent estimation of individual components, color, particle angularity and other descriptive criteria are available commercially.
Classification Group Name
An abbreviated summary of the USCS classification scheme is provided in Figure M-4 (after ASTM, 2006d). Soils are initially classified as “coarse-grained” or “fine-grained,” depending on the dominance of gravel and/or sand-size particles versus silt and/or clay-size particles. The term describing the dominant particle size is further modified based on the abundance of other particle sizes, with a code applied to each grouping. The classification scheme somewhat confusingly uses a minimum of 12% clay + silt to describe a sand or gravel as “with fines” but a minimum of 15% sand + gravel to describe a fine-grained soil as “with sand” or “with gravel.”
Figure M-4. USCS Soil Classification Scheme (after ASTM 2006d)
| Major Divisions | Code | Description | ||
| Coarse-Grained Soils More than 50% retained on a 0.075 mm (No. 200) sieve | Gravels 50% or more of course fraction retained through a 4.75 mm (No. 4) sieve | Clean Gravels (<5% fines) | GW | Well-graded gravels, sandy gravels or gravels with sand; little to no fines |
| GP | Poorly-graded gravels, sandy gravels or gravels with sand; little to no fines | |||
| Gravels with Fines (>5% fines) | GM | Sandy gravels with silt, gravels with sand and silt | ||
| GC | Sandy gravels with clay, gravels with sand and clay | |||
| Sands 50% or more of course fraction passes through a 4.75 mm (No. 4) sieve | Clean Sands (<5% fines) | SW | Well-graded sands, gravelly sands, or sands with gravel; little to no fines | |
| SP | Poorly-graded sands, gravelly sands, or sands with gravel; little to no fines | |||
| Sands with Fines (>5% fines) | SM | Silty sands, sands with silt | ||
| SC | Clayey sands, sands with clay | |||
| Fine-Grained Soils More than 50% passes through a 0.075 mm (No. 200) sieve | Silts and Clays | ML | Silt, sandy silt, clayey silt, silt with fine sand and clay | |
| CL | Clay, silty clay, clay with silt and fine sand | |||
| OL | Organic silt and clay (loam) | |||
| Highly Organic Soils | PT | Peat and other highly organic soils | ||
Notes:
- Gravel: >5.0mm to <75mm), sand: 0.075-5.0mm, silt: 0.005-0.075mm); clay: <0.005mm.
- Grading refers to the range of particle sizes in the soil. “Well-graded” gravels and sands contain a wide range of coarse particle sizes, poorly graded soils do not.
- Describe coarse-grained soils as “with fines” if >12% silt and clay; combine terms if between 5% and 12% fines
(e.g., GW-GM). Describe soil as “with sand” or “with gravel” if the soil contains >15% sand or gravel. - Silts and clays can be further defined in terms of liquid limit and plasticity as well as other criteria (see ASTM 2006d).
If the second-most dominant grain size makes up >30% of the soil type then include that grain size with the name. For example a soil composed of 30% fines and 75% sand is described as a “silty-clayey sand (SM).” If dominance of the fines by silt versus clay is known then a more specific code can be assigned, for example “SM” for a silty sand or “SC” for a clayey sand. Note that it can be difficult in the field to distinguish between fine sand, silt and clay without significant experience.
A sand with 12% to <30% silt and clay is described as “sand with fines (SM-SC).” Sands with 5% to 12% fines require dual symbols that include a description of grading, for example “well-graded sand with silt (SW-SM)”. A sand with <5% fines is simply described in terms of grading, for example “poorly graded sand (SP)”.
Fine-grained soils with >30% sand or gravel are described as “sandy” or “gravely.” Fine-grained soils containing 15% to <30% sand or gravel are described by adding “with sand” or “with gravel” to the group name (e.g., silt and clay with sand, ML-CL). Although not called for in the ASTM document referenced above, it is reasonable for the purposes of an environmental investigation to add “with sand” or “with gravel” to fine-grained soils that contain 5% to 15% coarse-grained particles. Dual classification of a soil type is appropriate if a sample has properties that do not distinctly place it into one group (e.g., SC/CL). Refer to the documents referenced above for additional soil descriptive terms.
Visual Estimation of Grain-Size Distribution
In practice the accurate classification of soils with a large fraction of fines can be challenging in the field without first drying and sieving the sample. Detailed analysis of particle size distribution is most accurately carried out in the laboratory if required as part of the investigation DQOs (e.g., Method D422; ASTM, 1998). As an alternative, field estimation of particle size distribution can be carried out in the following manner:
- Select a representative sample (Multi Increment sample preferred).
- Remove all gravel-size (>75mm or approximately three inches) or larger particles from the sample. Estimate and record the percent by volume of these particles. Only the fraction of the sample smaller than 75mm is classified.
- Estimate and record the percentage of gravel.
- Considering the rest of the sample, estimate and record the percentage of sand particles, typically the smallest particle visible to the unaided eye.
- Assign the remaining percentage to “fines”; do not attempt to separate silts from clays.
Estimate percentages to the nearest 5 percent. If one of the components is present in a quantity considered less than 5 percent, indicate its presence by the term “trace.” Percentage composition figures can assist in estimation of different size or particle type makeup of a sample. More precise lab methods might be required to accurately distinguish fine sand from silt and accurately determine clay composition if this information is required for completion of the field investigation.
Munsell Color
Color is described by hue and chroma using the Munsell Soil Color Chart (Munsell, 2000). For uniformity, the HEER Office recommends that investigators utilize this chart for soil color classification. This assists in comparisons of soils from different areas of a site or between sites. The Munsell Color Chart is a small booklet of numbered color chips with names like “5YR 3/4”, a specific type of reddish brown. The first part of the code (e.g., 5YR) describes the sample in terms of the basic color group (“hue”). The second part of the code (e.g., 3/4) describes the color in terms of lightness and darkness (“value”) and color intensity (“chroma”).
Descriptors should also note layering, mottling, gradation, or banding of colors. It is important to note and describe staining that might be related to contamination, particularly if the observation is correlated with other observations of odor, moisture, or appearance (e.g., presence of apparent petroleum liquids or green staining possibly related to contamination with copper-chromium-arsenic).
Consistency
Consistency describes the strength at which soil particles are held together. Descriptors include:
- Loose – Soil easily falls apart;
- Friable – Soil initially holds together but easily crushed with gentle pressure;
- Firm – Soil crushes under moderate pressure and resistance is noticeable;
- Very Firm – Strong pressure required to crush soil; difficult to accomplish with thumb and forefinger.
Soils that are loose and easily crumbled even when wet are usually indicative of a low clay content. Dry soil that is very firm is usually indicative of a moderate amount of clay.
Moisture Content
The moisture content of the soil should be described qualitatively using the following terms and the corresponding definitions:
- Dry – Absence of moisture, dry to the touch.
- Moist – No visible water but moisture is sufficient to bind soil matrix.
- Wet – Visible water, usually when soil is sampled from a water table. In other instances, the wetness may also indicate the presence of non-aqueous phase liquid (NAPL), if accompanied by strong odor or unusual liquid color or viscosity.
Submit samples to a laboratory for follow-up moisture analysis.
Staining, Odor and the Presence of Contamination
Unusual odors should be noted in logs and soil descriptions if detected when sampling, as they may be related to hydrocarbons, solvents, or other chemical contamination in the subsurface. Hydrocarbon odor may range from gasoline to lubrication oil. Contaminant-related odors also might have a distinctive smell of decaying vegetation or a stronger astringent or sweet odor that could be indicative of solvent compounds.
Use of a field organic vapor analyzer for screening purposes is recommended when volatile or semi-volatile organic contaminants are expected at a site (refer to Section 8). Direct inhalation should be avoided if odors suspected to be related to contaminants are detected. A health and safety plan should be prepared for sites where volatile or semi-volatile contaminants are anticipated. A respirator may be required under some circumstances, in additional to other personal protective equipment (e.g., gloves, tyvex suits, safety boots, etc.).
Note and describe staining or unusual moisture found in combination with unusual odors, as the combination of characteristics is often indicative of solvents, petroleum or other Non-Aqueous Phase Liquid (NAPL) contamination in soil (i.e., separate phase liquid not dissolved into water). The staining or moisture indicative of NAPL is often gray to brown in hue but can range in appearance from clear to completely black. Gasoline sometimes imparts a greenish to bluish hue to soil. Along with unusual odor and color, the moisture indicating NAPL contamination most often has an unusual textural aspect. For example, hydraulic fluids may feature a tacky or sticky feel accompanied by a sweet odor, while lubrication oil is most commonly much more viscous than water and accompanied by a dark color and a heavy, musky odor.
Appearance of coarser fragments
Angularity of coarser particles is often an indicator of mode of deposition. The following criteria describe the angularity of coarse sand and gravel particles:
- Rounded particles have smoothly-curved sides and no edges;
- Subrounded particles have nearly plane sides, but have well-rounded corners and edges;
- Subangular particles are similar to angular, but have somewhat rounded or smooth edges;
- Angular particles have sharp edges and relatively plane sides with unpolished surfaces. Freshly broken or crushed rock would be described as angular.
Note that both angular and rounded particles can be associated with depositions of volcanic ash.
Example Soil Descriptions
Description of coarse-grained soil samples
A coarse-grained soil is one that is primarily composed of sands and/or gravel particles. A soil is classified as a sand if greater than 50 percent of the coarse fraction is “sand-sized.” It is classified as a gravel if greater than 50 percent of the coarse fraction is composed of “gravel-sized” particles.
The written description of a coarse-grained soil should contain the following information:
- USCS classification name based on soil properties (particle size and percentage, plasticity, and other parameters as defined by USCS);
- Munsell color and color number;
- Moisture content;
- Relative density (if determinable);
- Coarse particle angularity and makeup by predominant particle type (e.g., coralline or basaltic).
An example coarse-grained sample description is presented below:
- POORLY SORTED SAND WITH SILT, medium- to coarse-grained, SW/SM (minor silt with approximately 80 percent coarse-grained sand-sized shell fragments, and 20 percent medium-grained basalt sand, and 5 percent to 15 percent ML), light olive gray, 5Y 6/2, moist, no odor, subrounded grains.
Description of fine-grained soil samples
Fine-grained soil is subdivided into clays and silts according to its plasticity. Clays are plastic while silts have little or no plasticity. The written description of a fine-grained soil should contain similar information as noted above, in addition to information on plasticity An example fine-grained sample description is presented below:
- SANDY CLAY WITH TRACE GRAVEL, CL (70 percent fines, 30 percent fine sand, with minor amounts of basalt gravel [< 5 percent]), light olive gray, 5Y 6/2, moist, faint odor, firm, moderately plastic.
M.1.4 Additional Sample Information
Additional sample information that might be required for a project includes moisture content, density/consistency, cementation, structure, and rock classification. A brief overview of these topics is provided below (see also Nielson 2006, US Navy 2007).
Density/Consistency (borings)
Density and consistency describe a physical property that reflects the relative resistance of a soil to penetration. The term “density” is commonly applied to coarse to medium-grained sediments (i.e., gravels, sands), whereas the term “consistency” is normally applied to fine-grained sediments (i.e., silts, clays). There are separate standards of measure for both density and consistency that are used to describe the properties of a soil.
The density or consistency of a subsurface soil is determined by observing the number of blows required to drive a standard 1 3/8-inch (35 mm) inner diameter (ID) split barrel sampler (commonly termed a standard penetrometer test [SPT] or terzaghi sampler) 18 inches using a drive hammer weighing 140 lbs (63.5 kilograms [kg]) dropped over a distance of 30 inches (0.76 meters). Record the number of blows required to penetrate each 6 inches of soil in the field boring log during sampling. The first 6 inches of penetration is considered to be a seating drive; therefore, the blow count associated with this seating drive is recorded, but not used in determining the soil density/consistency. The sum of the number of blows required for the second and third 6 inches of penetration is termed the “standard penetration resistance,” or the “N-value.” The observed number of blow counts must be corrected by an appropriate factor if a different type of sampling device is used (e.g., most commonly in Hawai‘i, a Modified California Sampler [MCS] with liners). For a 2-inch ID MCS equipped with brass or stainless steel liners and penetrating a cohesionless soil (sand/gravel), the N-value from the MCS must be divided by 1.43 to provide data that can be compared to the 1 3/8-inch ID SPT sampler data (University of Southern California 2001).
For a cohesive fine-grained soil (silt/clay), the N-value for the MCS should be divided by a factor of 1.13 for comparison with 1 3/8-inch ID SPT sampler data (US Navy 2007).
Drive the sampler and record blow counts for each 6-inch increment of penetration until one of the following occurs:
- A total of 50 blows have been applied during any one of the three 6-inch increments; a 50-blow count occurrence shall be termed “refusal” and noted as such on the boring log.
- A total of 150 blows have been applied.
- The sampler is advanced the complete 18 inches without the limited blow counts occurring, as described above.
If the sampler is driven less than 18 inches, record the number of blows per partial increment on the boring log. If refusal occurs during the first 6 inches of penetration, the number of blows will represent the N-value for this sampling interval. Table M-1 and Table M-2 present representative descriptions of soil density/consistency verses N-values.
Table M-1. Measuring Soil Density with Standard Penetration Test and Modified California Sampler – Sands, Gravels
| Description | Standard Penetration Test Sampler Field Criteria (N-Value) 1 3/8 in. ID Sampler |
Modified California Sampler Field Criteria (N-Value) 2 in. ID Sampler using 1.43 factor |
| Very Loose | 0–4 | 0–6 |
| Loose | 4–10 | 6–14 |
| Medium Dense | 10–30 | 14–43 |
| Dense | 30–50 | 43–71 |
| Very Dense | > 50 | > 71 |
Table M-2. Measuring Soil Density with a Standard and California Sampler – Fine Grained Cohesive Soil
| Description | Standard Penetration Test Sampler Field Criteria (N-Value) 1 3/8 in. ID Sampler |
Modified California Sampler Field Criteria (N-Value) 2 in. ID Sampler using 1.13 factor |
| Very Soft | 0–2 | 0–2 |
| Soft | 2-4 | 2-4 |
| Medium Stiff | 4-8 | 4–9 |
| Stiff | 8-16 | 9-18 |
| Very Stiff | 16-32 | 18-36 |
| Hard | > 32 | > 36 |
Cementation
Cementation is used to describe the friability of a soil. Cements are chemical precipitates that provide important information as to conditions that prevailed at the time of deposition, or conversely, diagenetic effects that occurred following deposition. Seven types of chemical cements are recognized by Folk (1980). They are as follows:
- Quartz – siliceous;
- Chert – chert-cemented or chalcedonic;
- Opal – opaline;
- Carbonate – calcitic, dolomitic, sideritic (if in doubt, calcareous should be used);
- Iron oxides – hematitic, limonitic (if in doubt, ferruginous should be used);
- Clay minerals – kaolinite, chlorite;
- Miscellaneous minerals – pyritic, collophane-cemented, glauconite-cemented, gypsiferous, anhydrite-cemented, baritic, feldspar-cemented, etc.
Of these, only 4 through 6 are commonly encountered in Hawaiian substrate.If the clay minerals are detrital or have formed by recrystallization of a previous clay matrix, they are not considered to be a cement. Only if they are chemical precipitates, filling previous pore space (usually in the form of accordion-like stacks or fringing radial crusts) should they be included as “kaolin-cemented,” “chlorite-cemented,” etc.The degree of cementation of a soil is determined qualitatively by utilizing finger pressure on the soil in one of the sample liners to disrupt the gross soil fabric. The three cementation descriptors are as follows:
- Weak – friable (crumbles or breaks with handling or slight finger pressure);
- Moderate – friable (crumbles or breaks with considerable finger pressure);
- Strong – not friable (will not crumble or break with finger pressure).
Structure
This variable is most appropriate to the vertical extent observed in borings and sometimes to lateral extent observed in trenches. The variable is used to qualitatively describe physical characteristics of soil that are important to incorporate into hydrogeological and/or geotechnical descriptions of soil at a site. Appropriate soil structure descriptors are as follows:
- Granular – spherically shaped aggregates with faces that do not accommodate adjoining faces;
- Stratified – alternating layers of varying material or color with layers at least 6 mm (1/4 inch) thick; note thickness;
- Laminated – alternating layers of varying material or color with layers less than 6 mm (1/4 inch) thick; note thickness;
- Blocky – cohesive soil that can be broken down into small angular or subangular lumps that resist further breakdown;
- Lensed – inclusion of a small pocket of different soil, such as small lenses of sand, should be described as homogeneous if it is not stratified, laminated, fissured, or blocky;
- Prismatic or Columnar – particles arranged about a vertical line, ped is bounded by planar, vertical faces that accommodate adjoining faces (prismatic has a flat top, columnar has a rounded top);
- Platy – particles are arranged about a horizontal plane.
Other sample appearance descriptors include:
- Mottled – soil that appears to consist of material of two or more colors in blotchy distribution;
- Fissured – breaks along definite planes of fracture with little resistance to fracturing (determined by applying moderate pressure to sample using thumb and index finger);
- Slickensided – fracture planes appear polished or glossy, sometimes striated (parallel grooves or scratches).
Rock Classification
The purpose of rock classification is to thoroughly describe the physical and if possible, mineralogical characteristics of a rock sample collected through rotary sampling, or significant rock fragments encountered in a soil matrix drilled into by hollow stem auger, or in a trench, and to classify it according to a common system. Because rock classification systems vary, and to date there is no universally accepted rock system equivalent to the soil USCS, the HEER Office recommends a general rock classification system similar to the US Navy standard operational procedure developed for description of rock types; however, it is modified because the Navy system includes rock types not found in the state of Hawai‘i (US Navy 2007).Rock descriptions preferably should be made by a trained geologist or geotechnical engineer. The items essential for classification include: Rock Name (e.g., coral limestone), Color (according to the Munsell code), Texture/Grain size (e.g. fine-grained, porphyritic), Structure (e.g., fractured, massive, porous), degree of weathering, and overall Classification according to the following general rock types:
- Conglomerate (CG) – Coarse-grained, consolidated sedimentary rock, including conglomerate and breccia.
- Sandstone (SS) – Consolidated sedimentary rock composed primarily of sand-sized particles.
- Mudstone (MS) – Consolidated sedimentary rock composed primarily of silt-sized or finer particles.
- Carbonates (LS) – Chemical or biological precipitates including coralline limestone, algal limestone, cemented shell limestone.
- Basalt (IE) – Although it is conceivable that very deep borings may encounter intrusive igneous rock, the predominant igneous rock that will be encountered in Hawai‘i is basalt. The description of basalt should include an identification of the encountered rock as predominantly a’a or pahoehoe. Descriptions of basalt should also have an indication of degree of weathering (if possible) and any identifiable dominant zones, depths, identifiable fracture orientations, clinker zones in a’a, or interconnected / elongated vesicles in either lava type. All of these may indicate preferential pathways for groundwater travel.
- Tuff (T) – Descriptions of tuff should include boring structure characteristics as described previously, degree of friability, and degree of weathering.
Where possible, rock types should also be identified according to depth range below ground surface. Descriptions of rock type should pay primary attention to characteristics that potentially affect groundwater behavior (e.g. basalt fracturing, carbonate porosity). An example rock description is as follows:
- Tuff (T), dusky red, 2.5 YR 3/2, welded, horizontal layers (~0.5 inch) of sand-sized ash/rock fragments grading upwards to laminated fine grained ash, 5 to 6 feet bgs, no fracturing with upper laminations highly weathered and altering to clay.
Other Subsurface Boring Soil/Rock Information
Optional but desirable associated information to accompany the boring logs would be photographs of boring locations, and for deeper borings, photographs of recovered samples. Each photograph should have a unique qualifying identification number or code. This code and some of the pertinent boring/soil sample information indicated on the list above should be written down and included within the photograph. A common method to include the information is to write it on paper or a reusable dry-erase type of board and to place the paper or board within the view of the photograph along with a common object (e.g., pen) for scale.
Return to the Top of the Page
M.2 EQUIPMENT PREPARATION/DECONTAMINATION
 Decontaminate sampling devices used to collect samples prior to use and between DUs. This includes drill rods and coring used for the collection of subsurface increments and samples. Decontamination of sampling equipment during the collection of increments within a DU for preparation of a bulk Multi Increment sample is not necessary. This includes the collection of samples or subsamples for the same DU layer, when multiple DU layers and core increments are being sampled (e.g. subsurface borings). Decontamination of equipment between DU replicates samples is recommended, since replicates are intended to be completely independent.
Decontaminate sampling devices used to collect samples prior to use and between DUs. This includes drill rods and coring used for the collection of subsurface increments and samples. Decontamination of sampling equipment during the collection of increments within a DU for preparation of a bulk Multi Increment sample is not necessary. This includes the collection of samples or subsamples for the same DU layer, when multiple DU layers and core increments are being sampled (e.g. subsurface borings). Decontamination of equipment between DU replicates samples is recommended, since replicates are intended to be completely independent.
Protect decontaminated equipment from incidental contact with potential contaminant sources by placing in sealed plastic bags or otherwise keeping the equipment well covered.
The following, triple-wash approach is recommended as a default procedure for decontamination of sampling equipment where trace levels of contaminants are being investigated (Figure M-5).
- Removed caked soil and debris from sampling equipment by hand;
- Wash with light detergent;
- Rinse with tap-water;
- Rinse a second time with tap-water.
The use of solvents to clean equipment should be avoided in order to minimize the generation of potential hazardous waste (see M.3 and USEPA 2015b). Document the decontamination procedure in the SAP and the final investigation report. These procedures may not be adequate for decontamination of equipment used to collect water samples (refer to Section 6). Carry multiple sets of sampling tools in order to expedite sample collection and allow decontamination of equipment in batches, ideally just once a day at the start or end of a sampling day.
Heavy equipment parts necessary for the advancement of any sampling device must be steam cleaned or high pressure/hot water washed between DU locations. Examples of these types of equipment include auger flights, drill rods, and backhoe buckets.
The collection of soil samples beneath concrete pads, floors, or asphalt paved areas may sometimes be necessary. If the equipment used to remove the concrete or asphalt has the potential to come into direct contact with the underlying soil, it must also be decontaminated. Decontaminate this equipment prior to and between sample locations in a manner similar to decontamination procedures discussed above for heavy equipment.
The collection and testing of equipment rinsate samples is not required or necessary for typical soil investigations. The practice is designed for “ultraclean” sampling approaches most typically associated with the collection of water samples, where parts-per-trillion level accuracy of laboratory data is desired. The collection of large Multi Increment soil (and sediment) samples also minimizes the potential for cross contamination of samples if small amounts of soil are inadvertently left on sampling tools, provided that the basic decontamination procedures described above are followed.
Return to the Top of the Page
M.3 INVESTIGATION DERIVED WASTE
Investigation-derived waste (IDW) generated during the collection of environmental samples must be properly managed and disposed of following completion of the investigation. Typical types of IDW include (after USEPA 2014f; see also USEPA 1991g, SERAS 1994):
- Personal protective equipment (PPE, e.g. disposable coveralls, gloves, booties, respirator canisters, splash suits, etc.)
- Disposable sampling equipment and related items (e.g., plastic trowels, core samplers, broken or unused sample containers, sample container boxes, tape, etc.);
- Soil cuttings from drilling or hand augering;
- Drilling mud or water used for mud or water rotary drilling;
- Decontamination wash water, rags, towels, etc.;
- Spent solvents used in sample preparation (e.g., methanol preservation) or cleaning;
- Other non-hazardous waste (plastic ground cloths, packing and shipping materials, etc.).
An effort should be made to minimize the amount of IDW generated during a site investigation to the extent practicable.
It is the responsibility of the property owner and the party conducting the sampling to properly dispose of all waste generated in accordance with local, state and federal regulations. If IDW is designated for disposal to a landfill or any other off-site location then the generator must make a Hazardous Waste Determination under RCRA (Resource Conservation and Recovery Act) and in accordance with Hawai‘i Administrative Rules (HAR) §11-262-11. Material that meets the regulatory classification as “hazardous waste” must be disposed of at a permitted, hazardous waste treatment, storage or disposal facility. There are currently no hazardous waste landfills in Hawai‘i. Therefore, IDW classifiable as hazardous waste must be disposed of at a regulated facility on the mainland.
A hazardous waste determination is a step-by-step process. First determine if the waste is specifically exempted by HAR §11-261-4. Petroleum-contaminated soil and materials are also excluded from hazardous waste regulations and can be disposed of at a municipal landfill, provided that it does not contain other contaminants which could cause it to be classifiable as hazardous waste. Wastes that are not specifically excluded are further assessed as part of the hazardous waste determination as follows (HAR §11-261-2):
- Listed Wastes: Specifically listed as a hazardous waste in HAR §11-261-2 (F-listed waste)
- Testing – Testing the waste for toxicity, ignitability, corrosivity, or reactivity according to the methods set forth in subchapter C of HAR §11-261; and/or
- Knowledge (e.g., known flammable solvent; see also Construction and Demolition Waste Disposal General Guidance (HDOH 2011j).
Waste that meet criteria for classification of “hazardous” under these methods must be disposed of at a permitted facility. Categorization as a “listed” hazardous waste primarily applies to pure product (e.g., some pesticides) or process wastes (e.g. spent methanol and other solvent) and is not generally applicable to IDW. Hazardous waste regulations most commonly apply to excess soil that fails a leaching test criteria for disposal in a municipal landfill, referred to as the Toxicity Characteristics Leaching Procedure (TCLP). These materials must be managed as hazardous waste and disposed of accordingly at a permitted facility.
Soil, including borehole cuttings, that is not classifiable as hazardous waste can be placed on site or disposed of as follows:
Soil meets Tier 1 EALs for unrestricted land use (e.g., residential):
- No restrictions on reuse provided that DU volume is <100 yd³ (see HDOH, 2017).
Soil fails Tier 1 EALs but meets commercial/industrial EALs and appropriate for current site use:
- Place within the area where the soil was collected (surface soil).
- Put back into the boring (subsurface cores);
- Place in an on-site disposal unit (any disposal unit exceeding one cubic yard should be discussed with the HDOH SHWB to evaluate if a permit is required);
- Transport to a HDOH-permitted off-site treatment/disposal facility.
Long-term management under an EHMP is required for soil that fails Tier 1 EALs for unrestricted reuse (or alternative, approved action levels) but is to be left on site (Section 13; see also HDOH 2007). Soil that fails Tier 1 EALs but meets commercial/industrial EALs should not be placed in otherwise clean areas of the site or taken offsite for reuse at another location.
For decontamination fluids:
- Pour onto ground in area where samples were collected to allow infiltration or evaporation;
- Transport to a HDOH-permitted off-site treatment/disposal facility.
Used disposable tools, PPE, waste rags, towels, packing material, ground cloths, etc., (maximum 100kg per site investigation in order to qualify for small quantity generator exemption; refer to USEPA 1991f, 2014).
- Double bag and dispose of in an on-site trash container, at a waste collection center, or a municipal landfill.
Always check with the landfill operators to determine their acceptance and testing requirements (if applicable) for non-hazardous IDW materials being disposed. The generation of hazardous IDW should be minimized. Most routine investigations should not produce any hazardous IDW. The use of solvents for cleaning of equipment should be minimized (USEPA, 2014). Solvent-free cleaning procedures for routine cleaning and decontamination as described in Section M.2 should be referred to (see also USEPA 2015). If the use of solvents is required, for example at sites impacted with tarry material, the volume should be minimized and mixing of waste solvent with detergent/wash water mixes avoided.
Management and disposal of waste groundwater generated during developing and purging activities is discussed in Section 6 and summarized below. Development and purge water can be disposed of on the ground immediately downgradient of the well provided that it is generated from the uppermost groundwater unit, is not impacted above action levels applicable to the site, does not contain free product or exhibit a sheen, and is not allowed to runoff into a surface water body or storm drain (refer to USEPA 2014). If these criteria cannot be met then the water must be disposed of at an offsite, regulated facility (e.g., municipal landfill or other treatment facility). Development and purge water should not be disposed in monitoring wells. Non-hazardous IDW such as drill cuttings, drilling mud, purge or development water, decontamination wash water, etc., should not be disposed of in dumpsters. [Note that guidance presented above replaces and supersedes guidance presented in the 2009 version of Section 6; scheduled for updates in 2017]
If knowledge of the contaminant or analytical testing determines the IDW falls under hazardous waste regulations then the material must either be (1) managed off-site at a permitted facility approved for the waste or (2) stored securely on-site in accordance with HAR §11-262-34, unless HAR §11-261-5 is applicable. Regulated hazardous waste left on-site must be handled in a fashion that does not pose an immediate threat to human health or the environment. The Solid and Hazardous Waste Branch (SHWB) should be contacted for concurrence with the manner of treatment or handling of IDW characterized as hazardous waste. The proximity of residents and workers in the surrounding area and site security must be considered before deciding to leave hazardous waste on site.
Return to the Top of the Page
M.4 FIELD WORK COMPLETION
The field investigation team should restore the investigation site once soil sampling and associated field investigation is completed to as close to pre-investigation condition as possible. This includes, but is not limited to the following:
- Demobilization of all equipment (e.g. drill rigs);
- Removal of field-related structures (e.g. temporary field trailers);
- Proper management or disposal of IDW;
- Repair of structural changes made to the site as a result of investigation (e.g., patching or resurfacing of concrete/asphalt surfaces cut for borings).
In particular, after any subsurface soil sampling is completed and if no other field investigation activity is to be conducted within a boring location (e.g., monitoring well installation, product removal) the borehole must be securely closed and sealed. If not properly closed and sealed, boreholes may otherwise act as a conduit for contamination. All procedures and materials for sealing boreholes should be outlined in the SAP for review prior to initiation of the field investigation. The procedures for closing and sealing boreholes recommended by the HEER Office follow those for abandoning and closing monitoring wells, as described in Section 6.9.
In some instances where follow-on activity at the site is planned but not yet initiated (e.g., subsequent soil removal) or if a potential exposure pathway or other hazard exists (e.g., unstable structure), retaining some structures such as temporary fencing or signage onsite is appropriate and should be implemented and documented in the site investigation report.
The HEER Office strongly recommends that site restoration as close as possible to pre-investigation conditions be documented in photographs, and the photographs be included as part of the site investigation report.
Return to the Top of the Page
APPENDIX N. COMMON INVESTIGATION ERRORS AND PROBLEMS
N.1 INAPPROPRIATELY SIZED DUS
The designation of Decision Units (DUs) for site characterization is discussed in Section 3.3. It is important to ensure that DUs are appropriately sized to meet site investigation objectives. Decision Units should ultimately be sized to address potential environmental hazards posed by contaminants in soil at the site. This always includes direct exposure and depending on the contaminant can also include leaching, gross contamination and other concerns (see Appendix B).
Direct exposure concerns under current site conditions are most directly evaluated through the designation of Exposure Area DUs. As discussed below, however, separate characterization of known or suspected areas of heavier contamination within an exposure area is still recommended. Leaching, gross contamination and other concerns are most directly evaluated based on Source Area DUs. The latter requires a more detailed understanding of the locations of potential heavy contamination based on the site history, field observations, and interviews with people knowledgeable of the site and related information. Source Area DUs are commonly a few hundred to a few thousand square feet in size and typically smaller than Exposure Area DUs that might be designated at the same site. The maximum size of a Source Area DU for characterization purposes is generally set to the maximum DU size likely to be acceptable for exposure areas. Multiple DUs might be required for characterization for very large source areas to assess both risk and remedial optimization concerns.
Failure to adequately identify and characterize suspect source areas at the beginning of an investigation can have several consequences. Foremost is the need to identify suspect source areas as a basic objective of an environmental investigation. If historical information or field observations suggest that contamination might be concentrated in a specific area of a site, then this area must be characterized separately from anticipated clean areas. The intentional inclusion of small areas of heavy contamination within large areas of otherwise clean soil for characterization can also cause the entire DU to fail and unnecessarily drive up cleanup costs.
Assume for example that an older building on a 5000 square foot lot is to be demolished and a new home constructed. The entire lot might be considered to represent a single, “Exposure Area” DU for evaluation of direct exposure risk. Soil around the perimeter of the existing house is, however, suspected to have been treated with Technical Chlordane (chlordane), widely used in the past as a termiticide. Exceptionally high concentrations of chlordane in this area could erroneously imply that the entire property is contaminated above soil action levels.
This highlights the need to characterize the house perimeter as a separate, Source Area DU, with the remaining area of the yard tested as an Exposure Area DU. The perimeter of the house will likely be flagged for potential direct exposure concerns. If the new house is to be constructed on the existing foundation then exposure to treated soil in this area can subsequently be minimized by placing gravel, landscaping or pavement around the perimeter.
Contamination associated with source areas can also extend below the depth of soil included in the original Exposure Area DU. This deeper soil could potentially be excavated during future redevelopment and spread out across the surface, resulting in a higher exposure area concentration of chlordane than estimated from the original investigation.
Significant disagreement between replicate samples can indicate the presence of a localized area of heavy contamination within an initially large DU. If this occurs and the resulting data are inadequate for decision making (refer to Appendix L), then the original DU should be subdivided into smaller DUs for re-characterization. This situation can be avoided for contaminants known to be subject to potential exceptionally high small-scale variability (e.g., lead shot, PCBs, etc.) by designating reasonably small DUs up front and increasing the number and/or mass of increments collected within a DU.
The use of inappropriately small DUs can also interfere with an efficient site investigation. Decision unit sizes are guided by the need to address risk and optimize remedial efforts. While a strong resolution of contaminated versus clean areas is desirable, the use of excessively small DUs to characterize an area is generally not beneficial and unnecessarily adds to the cost of the investigation.
Return to the Top of the Page
N.2 DATA GAPS BETWEEN SURFACE DUS AND SUBSURFACE DU LAYERS
Traditional discrete sampling methods require extrapolation of contaminant concentrations between individual sample points, where data are not available. As discussed in the Hawaii field study of discrete sample variability, extrapolation between discrete data points can be highly unreliable (see Brewer et al. 2017a, b). Under Decision Unit-Multi Increment Sample (DU-MI)S investigation approach, the data generated represent the mean contaminant concentration for a designated area rather than a single point. The use of adjoining DUs and subsurface DU layers minimizes gaps in data obtained for a site. This helps avoid the need for additional characterization should contamination be found as well as help optimize remedial actions. Data gaps for precise delineation of the lateral or vertical extent of a source area might be acceptable under some circumstances but should be reviewed and discussed on a site-by-site basis.
Boundary DUs surrounding suspect source areas of heavy contamination should ideally be placed immediately adjacent to the Source Area DU, with no gaps of untested soil present (see Section 3.3.3). Multiple rings of DUs might be advantageous in case inner DUs unexpectedly fail action levels. If gaps are unavoidable, for example due to buildings or other access limitations between source areas and anticipated clean areas, then contamination in the untested area of soil should be assumed to be similar to that identified for the primary source area unless additional information suggests otherwise.
The same need to minimize data gaps holds true for subsurface soil. Traditional discrete sampling of subsurface cores involved testing of soil at widely spaced intervals at depth below the ground surface (e.g., every 5 feet). Contamination was typically assumed to extend halfway between points where concentrations above and below action levels were reported. Under a DU-MIS investigation approach the entire depth of soil targeted for collection of a Multi Increment (MI) sample is divided into separate but adjoining, DU layers for representative sampling and characterization. Extrapolation across data gaps is not necessary or desirable.
Return to the Top of the Page
N.3 INADEQUATE NUMBER OF INCREMENTS AND BULK SAMPLE MASS
Sampling theory requires that a sample of adequate mass be collected from an adequate number of points within a targeted DU to capture and represent distributional heterogeneity within the DU and to estimate a reliable mean (refer to Appendix D).
Recall that the number of increments collected and the representativeness of the sampling methodology used is, in theory, independent of the size of the DU (refer to Section 3.6.2). The number of increments might vary somewhat based on the form of the contaminant (e.g., more for lead nuggets or PCB droplets) or other suspicions about the degree of contaminant heterogeneity but increasing increments in such cases would apply to both small and larger DUs as well. The number of increments might vary somewhat based on the form of the contaminant (e.g., more for lead nuggets or PCB droplets) or other suspicions about the degree of contaminant heterogeneity but increasing increments in such cases would apply to both small and larger DUs as well.
A minimum of 30 to 75+ increments per DU is recommended, with a default of 50 for sites where the nature of contamination is uncertain (refer to Section 3.6.2). If the target contaminant does not show an unusual degree of heterogeneity in the DU soil, then approximately 30-50 increments are typically adequate to determine a representative mean concentration (determined by the collection and analysis of field replicate samples). For contaminants or situations where there is a relatively high degree of contaminant heterogeneity in the DU, larger numbers of increment (and/or larger masses for increments) are typically needed to obtain representative mean values. The adequacy of the number and mass of increments included is tested through the collection of replicate samples (refer to Appendix L).
An adequate mass and number of increments to obtain a representative sample is required for both surface soil as well as subsurface soil, discussed below. If a less-than-recommended number of increments can be collected from a targeted DU, especially in the case of subsurface soil, then field replicate data is crucial to help evaluate the usefulness of the data for decision-making. In general, using fewer increments than recommended increases the likelihood that the data might not prove to be adequately representative. Any limitations of the data identified should be discussed in the investigation report, as well as the potential need for more reliable characterization in the future.
Some sampling guidance documents and training classes have suggested that increments initially collected from a DU be combined into smaller “sampling unit” subsets for separate testing to provide a better understanding of contaminant distribution variability within the DU. For example, a DU might be divided into four subareas with 8 increments collected from each “SU” and combined and tested separately. This approach suffers from several shortcomings. Most importantly, DUs should be appropriately sized to the desired scale of decision making at the start of the investigation. If better resolution might be needed for an initially large DU then the DU should simply be subdivided into smaller DUs with an MI sample of adequate mass and number of increments collected from each DU.
Testing of poor-quality samples from DUs when a proper number of increments could have been collected is wasteful of investigation resources and should be avoided. The resulting data cannot be assumed to be representative of the area where the combined increments were collected (see Brewer et al. 2017a, b). From a field perspective, the added time and cost to collect an adequate number of increments (e.g., 30 to 75+) from each smaller area is also negligible, especially given the importance of the resulting data in decision making.
Collecting an adequate mass of soil (e.g., 1 to 3 kg) is usually feasible for a project, as is the collection of an adequate number of increments from exposed, surface soil. The collection of a large number of increments from subsurface soil DU layers might not be practical, however, due to cost or access issues (see Section 3.6.2 and Appendix G). If this is the case, then limitations on the reliability of data should be clearly discussed in the investigation report. Replicate data from at least 10% of the DUs are especially important in such cases (refer to Appendix L).
Return to the Top of the Page
N.4 IMPROPER INCREMENT SPACING
Improper spacing of sample increments is a common mistake in DU-MIS investigations. Sample data are most reproducible when increment spaced equally apart in all directions, referred to as “systematic random.” This applies to both the both the “x” and “y” directions laterally and, in the case of subsurface borings, in the “z” direction (Figure N-1; refer to Section 3.6.2). This approach helps to ensure that the distributional heterogeneity of a contaminant within the DU is reliably captured and represented by the sample collected (refer to Appendix D).
Examples of less reliable increment spacing methods are depicted in Figure N-2. Simple random (Figure N-2a) and stratified random (Figure N-2b) strategies can under or over represent localized areas of elevated contamination and are not reliable for characterization of soil, sediment and other types of “infinite element” particulate media (refer to Appendix D).
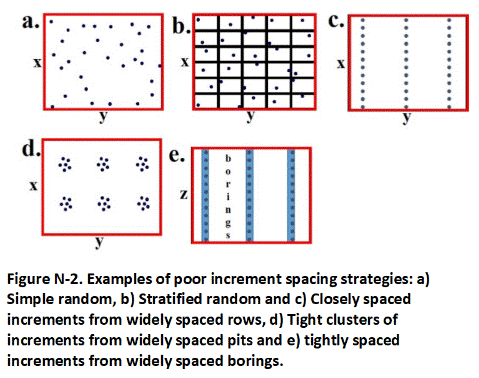
As a shortcut in the field, it can also be tempting to collect a large number of closely spaced increments from a few widely spaced lines (Figure N-2c) or single spots or pits (Figure N-2d) within a DU (examples c and d). These strategies will not provide an adequate coverage of the DU area and are especially prone to under estimation of the mean contaminant concentration for the DU as a whole.
The same potential for error holds true for the collection of closely spaced increments from widely spaced borings installed to collect samples from subsurface DUs (Figure N-2e). The vertical distance between increments collected within a single borehole should be approximately equal to the distance between the boreholes themselves. Since the thickness of DUs tends to be substantially less than the lateral length and width, the entire core extracted from a boring will in most cases represent a single increment (refer to Appendix G, Section G.3).
The only difference in comparison to the collection of increments from surface soils is that much larger sampling tubes (e.g., via a direct-push rig) are normally used to collect core increments from subsurface DU layers. The individual increments are normally too large for combination as a single sample. A subsample of each core increment must instead be prepared, for example by collection of 10, 5-gram, evenly spaced plugs of soil from a targeted DU layer interval. The plugs are subsamples of the core increment and do not represent individual increments themselves.
None of the increment spacing strategies depicted in Figure N-2 will produce reliable sample data and should be avoided. Poor increment spacing can cause replicate samples to fail and require re-characterization of the DU, wasting resources and unnecessarily extending the time and cost required to complete the project. Unreliable data can also pose future liability issues for the property owner.
Return to the Top of the Page
N.5 IMPROPER INCREMENT SHAPE
Gardening trowels are easy to use and decontaminate in the field for the collection of soil samples. Such tools are prone to collect wedge-shaped increments, however. This can bias the subsequent MI sample to the upper portion of the targeted DU layer, where the greater mass of soil was collected, and call into question the representativeness of the data in terms of the site investigation objectives. Note that this bias would not necessarily be reflected in replicates samples collected from the same DU, since the same error is carried forward in each individual sample.
Trowels should be avoided when tools that allow the collection of more core-shaped increments can be utilized (e.g., sampling tubes). A core-shaped increment is ideal since it equally represents the targeted DU layer in both the vertical and lateral direction (refer to Section 3.6.4 and Appendix F). The use of trowels and/or other tools might be unavoidable for hard-packed or gravely soils, however (see Appendix F). If this is the case, then an effort should be made to collect cylindrical-shaped increments that are equally representative of the full thickness of the DU. This approach might also be required for dry, loose soils that would otherwise fall out of sampling tubes or not be evenly extracted with drills or other coring equipment. Non-coring sampling alternatives might result in the collection of larger individual increment masses and larger bulk MI sample. This needs to be considered when planning the investigation and coordinating with the laboratory.
Return to the Top of the Page
N.6 MISUSE OF CO-LOCATED DISCRETE SAMPLES AND INCREMENT SPLITS
Field studies indicate that contaminant concentrations within a single sample or increment and co-located samples or increments can vary by orders of magnitude in an unpredictable and random manner (see Brewer et al. 2017a, b). The concentration of the contaminant in a simple subdivision of the discrete sample or increment (sometimes referred to as a split) or otherwise co-located sample/increment could well have no bearing on the concentration of the contaminant in the increment collected from the same location. Attempting to combine small groups of co-located increments into bulk MI sample for testing similarly poses the same risk of non-representativeness as described above.
Note also that replicate samples should not be collected from the same (or co-located with) initial increment locations (see Appendix L). While technically a separate sample, the precision of the MI sample data for a specific DU is accurately assessed by the collection of replicate samples from widely separated and completely independent locations.
Return to the Top of the Page
N.7 INADEQUATE LABORATORY PROCESSING
Inadequate processing of an MI sample negates the field representativeness of the sample and the validity of the resulting data. The resulting data reported by the laboratory can be considered to be no more useful than a single discrete sample collected from within the DU area.
It is important to ensure that the laboratory that receives the MI sample has a written standard procedure in place to properly process and collect a subsample for testing (refer to Appendix K). For non-volatile contaminants this includes drying, sieving and subsampling in accordance with sampling theory methodologies. Request a copy of the laboratory’s Standard Operating Procedure (SOP) for incremental sample processing and testing. Ideally the lab should be visited and the procedures used to manage MI samples demonstrated.
Return to the Top of the Page
N.8 INADEQUATE SUBSAMPLE MASS FOR ANALYSIS
The mass of soil collected in the field and extracted for analysis by a laboratory is dictated by sampling theory and must address both Fundamental Error associated with inherent, compositional and distributional heterogeneity and error in the physical collection of a representative subsample (see Appendix D). A minimum subsample mass for analysis of 10 to 30 grams is recommended for soil samples sieved to the <2 mm particle size (see Appendix K). When possible, a larger subsample mass is preferable to help further reduce the potential lab subsampling error and improve the precision of laboratory subsample replicates. Grinding (milling) of samples to a smaller particle size can allow for collection of a smaller lab subsample where appropriate for the contaminant or specified in a standard lab method (see Appendix K). Such cases should be discussed with the laboratory and the overseeing regulatory agency during sample investigation planning.
Standard laboratory methods for testing metals in soil only require one gram or less to meet analytical needs. The same is true for per- and per-and polyfluoroalkyl substances (PFASs). Unless the bulk sample has been ground, however, this is inadequate to ensure that the resulting data will be representative of the sample collected. The need to extract a larger mass of soil for metals analysis should be clarified with the laboratory prior to the initialization of field work.
Extraction of a larger subsample mass and/or grinding of the sample might be required if laboratory replicate samples indicate poor subsampling precision (see Appendix L). This should be discussed with the laboratory prior to submittal of the samples and procedures for retesting of samples included in the investigation work plan and instructions to the laboratory.
Return to the Top of the Page
N.9 LACK OF FIELD REPLICATE SAMPLE DATA
The need to collect replicate data might seem redundant with experience gained for a specific contaminant or a geographical area (see Appendix L). For example, 30-increment MI samples have been routinely demonstrated to generate reproducible data for are normally adequate to test for pesticides applied to agriculture fields or for soil contaminated by wastewater (refer to Section 3.6.2 and Appendix D). The representativeness of a DU sample can only be evaluated and documented if replicate samples are collected, however.
Routine collection of field replicates is required to demonstrate that correct sampling procedures were utilized (e.g., number of increments, systematic random sample spacing, correct increment shape and adequate sample mass, field handling/processing procedures, etc.).
The precision of MI sample data can decrease as the mean concentration of a contaminant increases. Unanticipated areas of localized contamination within DUs can also lead to decreased precision of normally acceptable MI samples. Field studies indicate that the concentration of a contaminant can vary by an order of magnitude or more in replicate samples collected from the same DU, even when an MI sample consists of greater than 50 increments (Brewer et al. 2017a, b). Under some circumstances even the higher recommended default of 75 increments per sample could be inadequate to demonstrate a representative mean contaminant concentration in a DU, such as when contaminants are distributed in a very heterogenic “nugget” form (e.g., lead pellets, or lead paint chips).
Testing of large numbers of discrete samples from a DU, for example with a portable XRF, can provide a semi- quantitative indication of the degree of small-scale variability within the DU and provide an indication of the relative number of increments necessary to collect a representative MI sample (e.g., greater number of increments needed for increasing heterogeneity). Statistical methods used to estimate the number of discrete samples needed to estimate the mean concentration of a contaminant within a DU (USEPA 2013b) are not, however, directly translatable to the number of increments required under an MI investigation and cannot be used as a substitute for the collection of replicate samples. This is due to multiple factors, including consistency in the manner in which the individual discrete samples were collected (e.g., shape, mass, etc.) and perhaps more importantly the mass of soil represented by each sample data point in comparison to the mass of soil typically represented by a single increment.
Return to the Top of the Page
N.10 REVERSION TO DISCRETE SAMPLING
Perhaps the most egregious error in site investigations is a reversion to discrete sampling due to real or perceived difficulties for the collection of proper MI sample in the field (refer to Appendix E). This is especially common for characterization of subsurface soil (see Appendix G). Sampling theory and the use of DU-MIS to characterize soil is not simply one alternative to past discrete sampling methods, it is a much needed replacement.
The concept of “DUs” was an inherent part of early, discrete soil sample investigation guidance (see Appendix C and Section 3.3). Discrete soil sample collection points were typically designated based on a desire to characterize contamination in one area versus another. As discussed below, the area intended to be represented by a single, discrete sample point (or cluster of sample points) is designated as a separate DU for characterization. A large-mass MI sample is then collected from multiple (e.g., 30- 75+) locations within this area rather than reliance on a small, discrete soil sample collected from a single location. The number of DUs designated for a particular investigation not coincidentally corresponds with the number of discrete soil samples or clusters of samples that might have been collected under past approaches.
The unreliability and inefficiency of discrete sample data remains the same regardless of the nature and location of the targeted soil. Consideration of sampling theory is still required to ensure that the resulting data are technically defensible and useful for decision making purposes. The fact that a targeted layer of soil is covered by additional soil that must first be penetrated for the collection of an MI sample cannot be used as a reason to revert to discrete sample collection approaches.
Targeted DU areas and layers, rather than single horizons, must always be designated as part of a site investigation regardless of the manner used to characterize the soil. As is the case for surface soil samples, subsurface samples must be of adequate mass and distribution within the DU to address fundamental error. Samples must also be processed at the laboratory in accordance with MI subsampling methods. If an ideal number of increments cannot be included in a DU layer sample due to access or cost limitations, then limitations regarding the reliability of the resulting data must be assessed and discussed based on a review of the replicate sample data. Identification of data limitations is also important where single borings are used for decision making purposes (see Appendix G).
Another error sometimes encountered in site investigations is a reversion to the collection of a single discrete sample when the targeted DU is very small, for example <100 Ft2 or even <10 ft2. Sampling theory is independent of DU area and volume (refer to Appendix C). A minimum, 1 to 3 kg sample must still be collected from the DU to address fundamental error. If collection of the recommended default number of increments from the DU is somehow not practical, then this should be noted and replicates collected and reviewed to determine precision of the sampling data. Any limitations identified through analysis of the replicate data should be discussed when reporting the results. The sample must be processed and subsampled for testing at the laboratory in accordance with MI sampling methods.
If the DU is so small that the entire volume of soil is to be collected and submitted to the laboratory, then processing and subsampling in accordance with MI sampling methods are still required (e.g., testing of sediment in a small sump). In this sense the soil submitted is not a true “sample” in terms of sampling theory, since the entire DU volume of interest is collected for analysis. The use of MI sampling methods to collect a representative sample from the DU in the field was not necessary. Any error in the resulting data would be fully attributable to laboratory subsampling and analysis errors since the entire mass is not being analyzed and a laboratory subsample must be collected.
Similar concerns and requirements as noted above also apply to the characterization of sediment that happens to be covered by a layer of water. Simplistic contouring between discrete sample points cannot be assumed to be reliable beyond the gross recognition of large contaminant patterns. DU layers, rather than single horizons should be designated and targeted for characterization (see Appendix G). Increments collected within a DU must be of adequate shape, number and mass to address fundamental error and generate a representative sample. It is possible that fewer numbers of increments might be adequate to collect a representative sample of sediment from designated DU areas, due to the nature in which the contaminant was released and the sediment deposited. This issue has not been evaluated in detail in the field to our knowledge, however. Limitations on the reliability of resulting data when an adequate number of increments cannot be collected must be discussed in the investigation report.
Return to the Top of the Page
APPENDIX O. DU-MIS INVESTIGATIONS UNDER TSCA
The investigation, cleanup, verification and disposal of soil contaminated with polychlorinated biphenyls (PCBs) is regulated under 40 CFR § 761.61 (PCB remediation waste) of the Toxic Substances Control Act (TSCA; USEPA 1998h). The Hawai‘i State Contingency Plan also authorizes HDOH to require the investigation and remediation of PCB-contaminated properties (refer to Section 2). This joint authority has caused problems as USEPA lags behind HDOH in the transition to multi increment sampling methods from outdated discrete sampling methods prescribed in 40 CFR 761.61(a) self-implementing on-site cleanup and disposal of PCB remediation waste and associated guidance documents (e.g., USEPA 1985, 1986). Discrete sample data are not allowed for final decision making at sites overseen by HDOH (refer to Appendix E). Error and uncertainty in discrete sample data are reviewed in a field study published by Brewer et al. (2017a, b).
Use of alternative procedures is provided for in 40 CFR 761.61(c)(1) risk-based disposal approval, subject to the approval of the USEPA Regional Administrator:
- Any person wishing to sample, cleanup, or dispose of PCB remediation waste in a manner other than prescribed in paragraphs (a) or (b) of this section … must apply in writing to the EPA Regional Administrator in the Region.
Responsible parties are encouraged to contact the TSCA office of USEPA Region IX when concentrations of PCBs in soil greater than 50 mg/kg are reported for MI samples. Under TSCA, soil with a concentration of >50 mg/kg PCBs must be disposed of at a hazardous waste landfill in the mainland US. Workplans for DU-MIS investigations at such PCB sites must be approved on a case-by-case basis by both HDOH and USEPA Region IX.
Of particular concern under TSCA is the need to minimize “dilution” of heavily contaminated soil with soil from surrounding, clean areas in sample data. Doing so might cause a conflict with Section 761.1(b)(5) of TSCA regulations, which states “No person may avoid any provision specifying a PCB concentration by diluting the PCBs, unless otherwise provided.” This concern can be avoided by designation of well-thought-out and researched Spill Area DUs at known or suspected PCB release sites in accordance with this guidance document and in coordination with HDOH. If PCB concentrations >50 mg/kg are identified in any DU then USEPA Region IX may also request to review and approve DUs designated for characterization of the site.
Dilution, as described under TSCA, can occur when samples intended to represent distinctly different areas (i.e., DUs) of a site are intentionally combined for a single analysis. The use of “composite” samples is also limited under TSCA regulations and guidance (e.g., USEPA 1985, 1986). As interpreted by HDOH, a Multi Increment sample is not a composite sample in the sense used in TSCA. A sample becomes a “composite” when soil from what should otherwise be separate DUs is combined. Under TSCA, each individual discrete sample is assumed to potentially represent an individual, PCB “contaminated zone” or “sampling area,” referred to in this guidance as “Spill Area DU” (see Section 3.3.2)(USEPA 1985):
- The PCB level is assumed to be uniform within (a contamination zone/spill area) and zero outside it.
The spacing of individual discrete samples was based in part on the anticipated size of a spill area in order to ensure that at least one sample was collected from each potential area (USEPA 1987):
- The decision maker must determine… the acceptable probability of not finding an existing contaminated zone in the suspected area. For instance, it might be determined that a 20 percent chance of missing a 100ft-by-100ft (10,000ft2) contaminated zone is acceptable but only a 5 percent chance of missing a 200ft-by-200ft (40,000ft2) zone is acceptable.
Under this scenario, TSCA regulations and associated guidance allow soil from multiple DU areas to be combined or “composited” into a single sample for analysis in order to reduce the total cost of laboratory analysis (Figure O-1; USEPA 1985, 1987, 1998h). This in effect allowed intentional “dilution” of suspect spill areas with surrounding areas of cleaner soil that should otherwise be separately characterized. The resulting data therefore had to be divided by the number of samples included in the composite, however, in order to ensure that no single “sampling area” exceeded the target cleanup level. A maximum of ten discrete samples was permitted to be included in a single composite, based on a target cleanup level of 10 mg/kg and a laboratory detection level of 1 mg/kg. Note that risk assessment guidance was still under preparation at the time that TSCA guidance and regulations were being prepared and the concept of “exposure areas” and risk were still not widely understood.
Under a more up-to-date, DU-MIS investigation, “compositing” in the sense initially intended under TSCA guidance would involve the intentional combination of Multi Increment samples collected from separate DUs into a single sample for testing. (Figure O-2) The resulting data would again need to be divided by the number of DUs and MI samples included in the composite, however, in order to ensure that no single DU area might exceed the target cleanup level.
Although this would save on analytical cost, compositing of MI samples is not allowed under HDOH guidance. An independent MI sample, representing what in the past might have been a single discrete sample, must instead be collected from each DU and individually tested for comparison against target action or cleanup levels. Intentional inclusion of suspect spill areas with anticipated clean areas for characterization as a single DU could be interpreted to violate the “anti-dilution” clause in TSCA regulations. For these reasons it is important to closely coordinate DU designation at PCB-release sites with HDOH and, as necessary, with USEPA Region IX.
As noted earlier, the intentional mixing of known or anticipated contaminated areas (i.e., “Spill Areas”) with clean areas as part of a site investigation is poor practice. Doing so risks unnecessarily increasing the area and volume of soil requiring removal or long-term management. Relatively small DUs, usually a few hundred to a few thousand square feet, should be designated for characterization within suspect spill areas (refer to Section 3.3.2). Perimeter DUs of a similar area and volume should be designated in anticipated clean areas around suspect spill areas. The maximum size of DUs in outer, anticipated clean areas should be limited to the size of current or anticipated exposure areas (default residential exposure area 5,000 ft2; see Section 3.3.1). These approaches will help ensure that the investigation and cleanup PCB-contaminated soil is carried out in an efficient and effective manner.
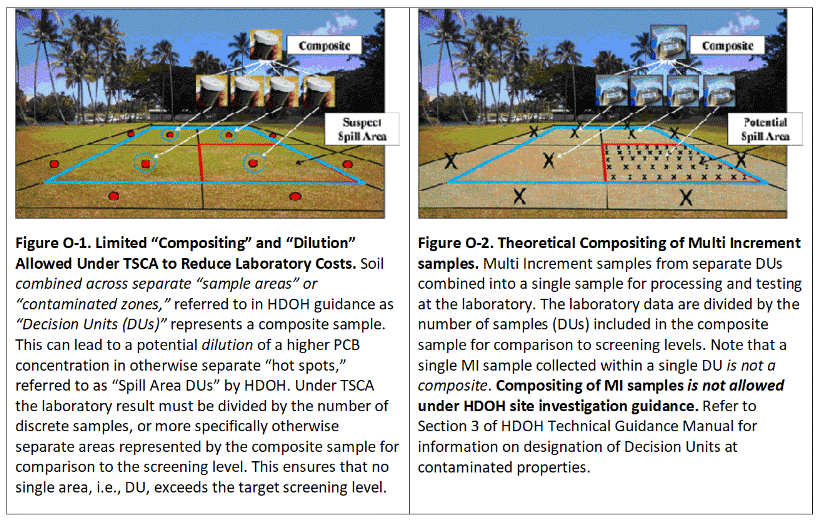
Return to the Top of the Page
APPENDIX P. CLEAN FILL GUIDANCE
Return to the Top of the Page
APPENDIX Q. REFERENCESAAFCO, 2015. GOODSamples: Guidance on Obtaining Defensible Samples: Association of American Feed Control Officials, October 2015, Champaign, Illinois. http://www.aafco.org/Portals/0/SiteContent/Publications/GOODSamples.pdfAAFCO, 2018. Good Test Portions: Guidance on Obtaining Defensible Test Portions: Association of American Feed Control Officials, June 2018, Champaign, Illinois. https://www.aafco.org/Portals/0/SiteContent/Publications/GoodTP_final_web.pdf?v3ASTM, 2003. Standard Guide for Laboratory Subsampling of Media Related to Waste Management Activities: American Society for Testing and Materials. D6323-98(2003). West Conshohokken, Pennsylvania: ASTM International. Website URL: http://www.astm.org/Standards/D6323.htm.ASTM, 2005. Standard Guide for Selection of Soil and Rock Sampling Devices Used With Drilling Rigs for Environmental Investigations: American Society for Testing and Materials. ASTM Standard D 6169-98(2005), ASTM International, West Conshohocken, Pennsylvania. Website URL: http://www.astm.org/Standards/D6169.htm.ASTM, 2006a. Standard Guide for Use of Hollow-Stem Augers for Geoenvironmental Exploration and Installation of Subsurface Water-Quality Monitoring Devices: American Society for Testing and Materials. ASTM Standard D 5784-95(2006), ASTM International, West Conshohocken, Pennsylvania. Website URL: http://www.astm.org/Standards/D5784.htm.ASTM, 2006b. Standard Guide for Use of Direct Air Rotary Drilling for Geoenvironmental Exploration and Installation of Subsurface Water-Quality Monitoring Devices: American Society for Testing and Materials. ASTM Standard D 5782-95(2006), ASTM International, West Conshohocken, Pennsylvania. Website URL: http://www.astm.org/Standards/D5782.htm.ASTM, 2006c. Standard Guide for Selection of Drilling Methods for Environmental Site Characterization: American Society for Testing and Materials. ASTM Standard D 6286-98(2006), ASTM International, West Conshohocken, Pennsylvania. Website URL: http://www.astm.org/Standards/D6286.htm.ASTM, 2008. Standard Practice for Rock Core Drilling and Sampling of Rock for Site Investigation: American Society for Testing and Materials. ASTM Standard D 2113-08, ASTM International, West Conshohocken, Pennsylvania. Website URL: http://www.astm.org/Standards/D2113.htm.ASTM, 2011. Standard Test Method for Standard Penetration Test (SPT) and Split-Barrel Sampling of Soils: American Society for Testing and Materials. ASTM Standard D1586-11, ASTM International, West Conshohocken, Pennsylvania. Website URL: http://www.astm.org/Standards/D1586.htmBrewer, R., J. Nagashima, M. Kelley, M. Heskett and M. Rigby, 2014, Evaluation of Total Petroleum Hydrocarbons in Vapor Intrusion Studies: International Journal Environmental Research and Public Health, Vol. 10, pp 2441-2467.Brewer, R., Peard, J. and M. Heskett. 2017a. A critical review of discrete soil sample reliability, Part 1 – Fieldstudy results: Soil and Sediment Contamination, Vol. 26 (1).Brewer, R., Peard, J. and M. Heskett. 2017b. A critical review of discrete soil sample reliability, Part 2 – Implications: Soil and Sediment Contamination, Vol. 26 (1).CalEPA, 2004. Guidance on the Implementation of EPA Method SW-846 5035: California Environmental Protection Agency, Department of Toxic Substances Control. November 2004.CAEPA, 2013. Preliminary Endangerment Assessment Guidance Manual: California Environmental Protection Agency, Department of Toxics Substances Control. October 2013.Esbensen, K., 2020. Introduction to the Theory and Practice of Sampling: IM Publications, ISBN: 978-1-906715-29-8.Gilbert, R.O., 1987. Statistical Methods for Environmental Pollution Monitoring. New York: Van Nostrand Reinhold Company Inc.HDOH, 2015a. Small-Scale Variability of Discrete Soil Sample Data, Part 1: Field Investigation of Discrete Sample Variability: Hawaii Department of Health, Hazard Evaluation and Emergency Response. June 2015.Website URL: http://eha- web.doh.hawaii.gov/eha-cma/Org/HEER/.HDOH, 2015b. Small-Scale Variability of Discrete Soil Sample Data, Part 2: Causes and Implications for Use in Environmental Investigations: Hawaii Department of Health, Hazard Evaluation and Emergency Response. June 2015. Website URL: http://eha- web.doh.hawaii.gov/eha-cma/Org/HEER/.HIDOH, 2016, Technical Guidance Manual (and updates): Hawaii Department of Health, Office of Hazard Evaluation and Emergency Response.HIDOH, 2017. Screening for Environmental Concerns at Sites with Contaminated Soil and Groundwater (and updates): Hawaii Department of Health, Office of Hazard Evaluation and Emergency Response.HIDOH, 2017b. Use of laboratory batch tests to evaluate potential leaching of contaminants from soil: Hawaii Department of Health, Office of Hazard Evaluation and Emergency Response.HIDOH, 2018. Collection and Use of Total Petroleum Hydrocarbon Data for the Risk-Based Evaluation of Petroleum Releases – Example Case Studies: Hawaii Department of Health, Hazard Evaluation and Emergency Response Office. August 2018.Hewitt, A.D., 1999. Frozen Storage of Soil Samples for VOC Analysis. Environmental Testing and Analysis, Vol. 8 (5): 18-25. Sept/Oct 1999.ITRC, 2018. TPH Risk at Petroleum Contaminated Sites: Interstate Technology and Regulatory Council, November 2018.Juhasz, A.L., Smith, E., Weber, J., Rees, M., Rofe, A., Kuchel, T., Sansom, L. and R. Naidu, 2007. Comparison of in vivo and in vitro methodologies for the assessment of arsenic bioavailability in contaminated soils. Chemosphere, Vol. 69, pp. 961-966.Kelley, M.E., Brauning, S.E, Schoof, R.A. and M.V. Ruby, 2002. Assessing Oral Bioavailability of Metals in Soil: Battelle Press, Ohio.MADEP, 2002. Characterizing Risks Posed by Petroleum Contaminated Sites: Massachusetts Department of Environmental Protection, Policy #WSC-02-411. October 2002. Website URL: http://www.mass.gov/eea/waste-mgnt-recycling/.Minnitt et al., 2007 Minnitt, R.C.A., Rice, P.M. and C. Spangenberg. Part 1: Understanding the components of the fundamental sampling error: a key to good sampling practice: The Journal of the Southern African Institute of Mining and Metallurgy, Vol. 107. August 2007.NJDEP, 2005. Field Sampling Procedures Manual: New Jersey Department of Environmental Protection, August 2005.Nielsen, David M., 2006. Environmental Site Characterization and Ground-Water Monitoring. Second Edition.Pitard, F.F., 2005. Sampling and Blending Conference Sunshine Coast, Queensland, Australia. Sampling Correctness – A Comprehensive Guideline. Might 9-12, 2005.Pitard, F.F., 2009. Fourth World Conference on Sampling & Blending, The Southern African Institute of Mining and Metallurgy. Theoretical, practical and economic difficulties in sampling for trace constituents.Pitard, F.F., 2019. Pierre Gy’s Sampling Theory and Sampling Practice, 3rd Edition. Boca Raton, FL: CRC Press, Inc.Ramsey, C. and A. Hewitt, 2005. A Methodology for Assessing Sample Representativeness, Environmental Forensics 6:71-75. Website URL: http://www.environmentalforensics.org/journal/volume6/number2.htm.Ruby, M.V., Davis, A., Schoof, R., Eberle, S. and C.M. Sellstone, 1996. Estimation of Lead and Arsenic Bioavailability using a Physiologically Based Extraction Test: Environmental Science Technology, Vol. 30, pp 422-430.Ruby, M.V., 2001. In Vitro Method for Determination of Lead and Arsenic Bioaccessibility (Standard Operating Procedure): Solubility Bioavailability Research Consortium, SOP 110499.SBRC, 1999. Standard Operating Procedure, In Vitro Method for Determination of Lead and Arsenic Bioavailability: Solubility/Bioavailability Research Consortium, Document 8601102.001 0601 1099 RN01 (contact Michael Ruby, Exponent, Boulder, Colorado or Dr. John Drexler, University of Colorado-Boulder, Department of Geological Sciences)TNRCC, 2002. Guidance on the Use of EPA Method SW-846 5035: Texas Natural Resource Conservation Commission. June 2002.USACE, 2001. Geotechnical Investigations: United States Army Corps of Engineers, Manual 1110-1-1804。January 2001.USACE, 2007. Protocols for Collection of Surface Soil Samples at Military Training and Testing Ranges for the Characterization of Energetic Munitions Constituents: United States Army Corps of Engineers. ERDC/CRREL TR-07-10. July 2007.USEPA, 1985. Verification of PCB Spill Cleanup by Sampling and Analysis: United States Environmental Protection Agency, Office of Toxic Substances, Washington D.C., August 1985. EPA-560/5-85·026.USEPA, 1986. Field Manual for Grid Sampling of PCB Spill Sites to Verify Cleanups: United States Environmental Protection Agency, Office of Toxic Substances, May 1986. EPA-560/5-86·017.USPEA, 1987. Data Quality Objectives for Remedial Response Activities: United States Environmental Protection Agency, Office of Solid Waste and Emergency Response, March 1987. EPA/540/G-87/003.USEPA, 1988a. Superfund Exposure Assessment Manual: United States Environmental Protection Agency, Office of Remedial Response, April 1988. EPA/540/1-881001.USEPA, 1988b. Guidance for Conducting Remedial Investigations and Feasibility Studies Under CERCLA: United States Environmental Protection Agency, Office of Solid Waste and Emergency Response, October 1988. EPA/540/G-891/004, OSWER Directive 9355.3 01.USEPA, 1989a. Methods of Evaluating the Attainment of Cleanup Standards, Volume 1: Soils and Solid Media: United States Environmental Protection Agency, Office of Solid Waste and Emergency Response, February 1989. EPA 230/02-89-042.USEPA. 1989b. Soil Sampling Quality Assurance User’s Guide: United States Environmental Protection Agency, Environmental Monitoring Systems Laboratory, March 1989. EPA/600/8-69/046.USEPA. 1989c. Risk Assessment Guidance for Superfund, Volume I, Human Health Evaluation Manual (Part A): United States Environmental Protection Agency, Office of Policy, Planning, and Evaluation, December 1989. EPA/540/1-89/002.USEPA. 1989d. Risk Assessment Guidance for Superfund, Volume II, Environmental Evaluation Manual: United States Environmental Protection Agency, Office of Policy, Planning, and Evaluation, March 1989. EPA/540/1-89/001.USEPA. 1991. Guidance for Data Usability in Risk Assessment (Part A): United States Environmental Protection Agency, Office of Research and Development, December 1991. EPA/540/R-92/003.USEPA. 1992. Preparation of Soil Sampling Protocols: Sampling Techniques and Strategies: United States Environmental Protection Agency, Office of Research and Development, July 1992. (EPA/600/R-92/128).USEPA, 1995. Superfund Program, Representative Sampling Guidance, Volume 1: Soil. Interim Final. United States Environmental Protection Agency, Office of Solid Waste and Emergency Response, December 1995. EPA 540/R-95/141, OSWER Directive 9360.4-16, PB96-963207.USEPA, 1998h. U.S. Environmental Protection Agency, Code of Federal Regulations. CFR Part 761.61, PCB Remediation Waste.USEPA, 2000. Data Quality Objectives Process for Hazardous Waste Sites: Final Guidance: United States Environmental Protection Agency, Office of Environmental Information, January 2000. EPA/600/R-00/007.USEPA, 2002a. Method 5035A. Closed-System Purge-and-Trap and Extraction for Volatile Organics in Soil and Waste Samples: United States Environmental Protection Agency, Office of Solid Waste, July 2002.USEPA, 2002b.A Review of the Reference Dose and Reference Concentration Process: United States Environmental Protection Agency, Risk Assessment Forum, December 2002. EPA/630/P-02/002F.USEPA, 2002c. Guidance on Environmental Data Verification and Data Validation. United States Environmental Protection Agency, Office of Environmental Information, November 2002. EPA/240/R-02/004.USEPA, 2003a. Guidance for Obtaining Representative Laboratory Analytical Subsamples from Particulate Laboratory Samples, United States Environmental Protection Agency, Office of Research and Development. November 2003. EPA/600/R-03/027.USEPA, 2003b. Sample Holding Time Reevaluation: United States Environmental Protection Agency, National Exposure Research Laboratory, Environmental Sciences Division, October 2003. EPA/600/R-05/124.USEPA, 2005. Guidelines for Carcinogen Risk Assessment: United States Environmental Protection Agency, Risk Assessment Forum, March 2005. EPA/630/P-03/001F.USEPA, 2006. Nitroaromatics, Nitramines, and Nitrate Esters by High Performance Liquid Chromatography (HPLC): United States Environmental Protection Agency, SW846 Methods, Method 8330B.USEPA, 2011. Exposure Factors Handbook: United States Environmental Protection Agency, Office of Research and Development, September 2011. EPA/600/R- 090/052F.USEPA, 2013. ProUCL Version 5.0.00 User Guide: United States Environmental Protection Agency, Office of Research and Development, September 2013. EPA/600/R- 07/041.USEPA, 2014. Hot Spots: Incremental Sampling Methodology (ISM) FAQs: United States Environmental Protection Agency, Superfund, March 2014.USEPA, 2019a. Leaching Environmental Assessment Framework (LEAF) How-To Guide: United States Environmental Protection Agency, Office of Land and Emergency Management and Office of Research and Development, Might 2019.USEPA, 2019b. Screening Levels for Chemical Contaminants: United States Environmental Protection Agency, prepared by Oak Ridge National Laboratories. November 2019.US Navy, 2015. Projects Procedures Manual (Procedure I-B-1 Soil Sampling): United States Department of the Navy, Environmental Restoration Program, Naval Facilities Engineering Command Pacific, May 2015.USNLM, 2016. National Center for Biotechnology Information. Phenylmercuric Acetate. United States National Library of Medicine. PubChem Open Chemistry Database. (Accessed June 2, 2016)USGS, 2005. National Field Manual for the Collection of Water-Quality Data (Book 9, Handbooks for Water-Resources Investigations, Chapter A8, Bottom-Material Samples): United States Geological Survey, June 2005.
| Primary Sources | Primary Release Mechanism | Secondary Sources | 1Potential Environmental Hazards | 2Hazard Present Under Current or Future Site Conditions? | Comments | |||||||||||||||||||||||||||||
| Current | Future | |||||||||||||||||||||||||||||||||
| ASTs, USTs, pipelines, drums, disposal areas, etc. | Spills, leaks, improper disposal | Soil | 3Risk to Human Health | Direct Exposure | YES | YES | ||||||||||||||||||||||||||||
| Vapor Intrusion into Buildings | YES | YES | ||||||||||||||||||||||||||||||||
| 4Risk to Terrestrial Ecological Habitats | YES | YES | ||||||||||||||||||||||||||||||||
| 5Leaching | YES | YES | ||||||||||||||||||||||||||||||||
| 6Gross Contamination | YES | YES | ||||||||||||||||||||||||||||||||
| Groundwater | 7Risk to Human Health | Direct Exposure | YES | YES | ||||||||||||||||||||||||||||||
| Vapor Intrusion into Buildings | YES | YES | ||||||||||||||||||||||||||||||||
| 8Risk to Aquatic Ecological Habitats | YES | YES | ||||||||||||||||||||||||||||||||
| 9Gross Contamination | YES | YES | ||||||||||||||||||||||||||||||||
| Primary Sources | Primary Release Mechanism | Secondary Sources | 1Potential Environmental Hazards | 2Hazard Present Under Current or Future Site Conditions? | ||||||||||||||||||||||||||||||
| ASTs, USTs, pipelines, drums, disposal areas, | Spills, leaks, improper disposal | Soil | 3Risk to Human Health | Exposure Type | Secondary Release Mechanism | Exposure Route | Receptors | |||||||||||||||||||||||||||
| On-Site Workers | Offsite Residents | Construction Workers | ||||||||||||||||||||||||||||||||
| Current/Future | Current/Future | Current/Future | ||||||||||||||||||||||||||||||||
| Direct Exposure | none | Ingestion | *No | *No | *No | *No | Yes | Yes | ||||||||||||||||||||||||||
| Dermal | *No | *No | *No | *No | Yes | Yes | ||||||||||||||||||||||||||||
| Dust/Vapors | Inhalation | *No | *No | *No | *No | Yes | Yes | |||||||||||||||||||||||||||
| 4Vapor Intrusion into Buildings | Vapors | Inhalation | Yes | Yes | No | No | na | na | ||||||||||||||||||||||||||
| 5Risk to Terrestrial Ecological Habitats | Current – No (no habitat or receptors) | Future – No (no habitat or receptors) | ||||||||||||||||||||||||||||||||
| 6Leaching | Current – Yes (soil in contact with groundwater) | Future – Yes | ||||||||||||||||||||||||||||||||
| 7Gross Contamination | Current – Yes (potential explosive vapors) | Future – Yes (potential explosive vapors) | ||||||||||||||||||||||||||||||||
| Ground-water | 8Risk to Human Health | Exposure Type | Secondary Release Mechanism | Exposure Route | Receptors | |||||||||||||||||||||||||||||
| On-Site Workers | Offsite Residents | Construction Workers | ||||||||||||||||||||||||||||||||
| Current or Future | Current or Future | Current or Future | ||||||||||||||||||||||||||||||||
| Direct Exposure | none | Ingestion | No | No | No | No | Yes | Yes | ||||||||||||||||||||||||||
| Dermal | No | No | No | No | Yes | Yes | ||||||||||||||||||||||||||||
| Inhalation | No | No | No | No | Yes | Yes | ||||||||||||||||||||||||||||
| 4Vapor Intrusion into Buildings | Vapors | Inhalation; | Yes | Yes | No | No | na | na | ||||||||||||||||||||||||||
| 9Risk to Aquatic Ecological Habitats | Current – *No (monitoring shows plume not migrating) | Future – *No (monitoring shows plume not migrating) | ||||||||||||||||||||||||||||||||
| 10Gross Contamination | Current – Yes (free product present) | Future – Yes (while free product present) | ||||||||||||||||||||||||||||||||
| Primary DU Area |
Final DU ID Number |
Sediment Type | Area | Sediment Interval | Estimated In Situ Sediment Volume |
*Estimated In Situ Sediment Volume |
||||||||||||||||||||||||||||
| DU-1 | DU-1A | Clayey Silt | 175,000 ft2 | 0.0 – 1.5 ft | 9,700 cyds | 7,300 cyds | ||||||||||||||||||||||||||||
| DU-1B | Clayey Silt | 175,000 ft2 | 1.5 – 3.0 ft | 9,700 cyds | 7,300 cyds | |||||||||||||||||||||||||||||
| DU-1C | Clayey Silt | 175,000 ft2 | 3.0 – 5.0 ft | 13,000 cyds | 9,700 cyds | |||||||||||||||||||||||||||||
| DU-2 | DU-2A | Clayey Silt | 150,000 ft2 | 0.0 -1.5 ft | 8,300 cyds | 6,250 cyds | ||||||||||||||||||||||||||||
| DU-2B | Clayey Silt | 150,000 ft2 | 1.5 – 3.0 ft | 8,300 cyds | 6,250 cyds | |||||||||||||||||||||||||||||
| DU-3 | DU-3A | Clayey Silt | 250,000 ft2 | 0.0 – 1.5 ft | 13,900 cyds | 10,400 cyds | ||||||||||||||||||||||||||||
| DU-3B | Clayey Silt | 250,000 ft2 | 1.5 – 3.0 ft | 13,900 cyds | 10,400 cyds | |||||||||||||||||||||||||||||
| DU-4 | DU-4A | Sand | 40,000 ft2 | 0.0 -1.5 ft | 2,200 cyds | 2,200 cyds | ||||||||||||||||||||||||||||
| DU-4B | Sand | 40,000 ft2 | 1.5 – 3.0 ft | 2,200 cyds | 2,200 cyds | |||||||||||||||||||||||||||||
| DU-4C | Sandv | 40,000 ft2 | 3.0 – 5.0 ft | 3,000 cyds | 3,000 cyds | |||||||||||||||||||||||||||||
| Total: | Total: 84,200 yds | 65,000 cyds | ||||||||||||||||||||||||||||||||
| Factors | Mining Industry | Environment Industry | ||||||||||||||||||||||||||||||||
| Investigation Target: | Commodities | Contaminants | ||||||||||||||||||||||||||||||||
| Example Media: | Crushed Ore | Soil and Sediment | ||||||||||||||||||||||||||||||||
| Field Characterization: | Designation of Lots | Designation of Decision Units (DUs) | ||||||||||||||||||||||||||||||||
| Basis: |
|
|
||||||||||||||||||||||||||||||||
| Sample Collection: | Multi Increment Samples | Multi Increment Samples | ||||||||||||||||||||||||||||||||
| 1Typical Sample Mass: | 100s to 1,000 kilograms | 1 to 3 kilograms | ||||||||||||||||||||||||||||||||
| 2Quality Control: |
|
|
||||||||||||||||||||||||||||||||
| 3Required Replicate Data Precision: | RSD <1-5% | RSD <35% | ||||||||||||||||||||||||||||||||
Notes:
|
||||||||||||||||||||||||||||||||||
| Study Site | Range Mean (mg/kg) | Range 95% UCL (mg/kg) | ||||||||||||||||||||||||||||||||
| Site A (arsenic) | 218 to 395 | 278 to 535 | ||||||||||||||||||||||||||||||||
| Site B (lead) | 170 to 356 | 215 to 469 | ||||||||||||||||||||||||||||||||
| Site C (PCBs) | 5 to 1,025 | 9.4 to >1,000,000 | ||||||||||||||||||||||||||||||||
| Targeted Contaminants of Concern | Receiving Site Land Use Category | 1Assumed Fill Reuse Area and Thickness | 2,3Default Stockpile Decision Unit Volume | Example COPCs | Primary Potential Environmental Hazards | |||||||||||||||||||||||||||||
| 4Volatile Compounds | Any | Area: 100-200 m2 Thickness: 0.5 m | 50-100 m3 | TPHg, TPHd, BTEX, naphthalene, PCE, TCE, mercury | Vapor intrusion, leaching | |||||||||||||||||||||||||||||
| 4,5Highly Leachable, Non‐Volatile Contaminants | Any | Area: 100-500 m2 Thickness: 0.5 m | 50-250 m3 | chlorinated herbicides, perchlorate, PFASs, explosives | Leaching and surface runoff or groundwater contamination | |||||||||||||||||||||||||||||
| 5,6,7Low Mobility Contaminants | Unrestricted Use | Area: 400-1,000 m2 Thickness: 0.25 m | 100-250 m3 | heavy metals, dioxins/furans, PCBs, PAHs, TPHo, organochlorine pesticides | Direct exposure | |||||||||||||||||||||||||||||
| Schools and High‐Density Residential Developments | Area: 1,000-2,000 m2 Thickness: 0.25 m | 250-500 m3 | ||||||||||||||||||||||||||||||||
| 8Parks and athletic fields contamination) | Area: 2,000-4,000 m2 Thickness: 0.25 m | 500-1,000 m3 | ||||||||||||||||||||||||||||||||
| 9,11Commercial/Industrial use only (localized fill source from previously developed area; assumed low-moderate risk of contamination) | Area: 2,000-4,000 m2 Thickness: 0.25 m | 500-1,000 m3 | ||||||||||||||||||||||||||||||||
| 10,11Commercial or Industrial use only (large, agricultural field, undeveloped land or dredged sediment fill source assumed low risk of contamination) | Area: 4,000-10,000 m2 Thickness: 0.25 m | 1,000-2,500 m3 | ||||||||||||||||||||||||||||||||
| CHEMICAL PARAMETER | 1Physical State | Molecular Weight | 2Vapor Pressure
mm Hg (25C) |
Henry’s Law Constant(H) (atm-m³/mol) |
||||||||||||||||||||||||||||||
| VOLATILE CHEMICALS Preserve Samples in Methanol in the Field (or approved alternative, see text) (VP>1 and Molecular Weight <200) |
||||||||||||||||||||||||||||||||||
| ACETONE | V | L | 58 | 2.3E+02 | 3.9E-05 | |||||||||||||||||||||||||||||
| BENZENE | V | L | 78 | 9.5E+01 | 5.61E-03 | |||||||||||||||||||||||||||||
| BIS(2-CHLOROETHYL)ETHER | V | L | 143 | 1.6E+00 | 1.7E-05 | |||||||||||||||||||||||||||||
| BROMODICHLOROMETHANE | V | L | 164 | 5.0E+01 | 2.1E-03 | |||||||||||||||||||||||||||||
| BROMOFORM | V | S | 253 | 5.4E+00 | 5.4E-04 | |||||||||||||||||||||||||||||
| BROMOMETHANE | V | G | 95 | 1.6E+03 | 6.3E-03 | |||||||||||||||||||||||||||||
| CARBON TETRACHLORIDE | V | L | 154 | 1.2E+02 | 2.7E-02 | |||||||||||||||||||||||||||||
| CHLOROBENZENE | V | L | 113 | 1.2E+01 | 3.2E-03 | |||||||||||||||||||||||||||||
| CHLOROETHANE | V | G | 65 | 1.0E+03 | 1.1E-02 | |||||||||||||||||||||||||||||
| CHLOROFORM | V | L | 119 | 2.0E+02 | 3.7E-03 | |||||||||||||||||||||||||||||
| CHLOROMETHANE | V | G | 50 | 4.3E+03 | 8.8E-03 | |||||||||||||||||||||||||||||
| CHLOROPHENOL, 2- | V | L | 129 | 2.5E+00 | 1.1E-05 | |||||||||||||||||||||||||||||
| DIBROMOCHLOROMETHANE | V | S | 208 | 5.5E+00 | 7.8E-04 | |||||||||||||||||||||||||||||
| DIBROMOETHANE, 1,2- | V | S | 188 | 1.1E+01 | 6.6E-04 | |||||||||||||||||||||||||||||
| DICHLOROBENZENE, 1,2- | V | L | 147 | 1.4E+00 | 1.9E-03 | |||||||||||||||||||||||||||||
| DICHLOROBENZENE, 1,3- | V | L | 147 | 2.2E+00 | 1.9E-03 | |||||||||||||||||||||||||||||
| DICHLOROBENZENE, 1,4- | V | S | 147 | 1.7E+00 | 2.4E-03 | |||||||||||||||||||||||||||||
| DICHLOROETHANE, 1,1- | V | L | 99 | 2.3E+02 | 5.6E-03 | |||||||||||||||||||||||||||||
| DICHLOROETHANE, 1,2- | V | L | 99 | 7.9E+01 | 1.2E-03 | |||||||||||||||||||||||||||||
| DICHLOROETHYLENE, 1,1- | V | L | 97 | 6.0E+02 | 2.7E-02 | |||||||||||||||||||||||||||||
| DICHLOROETHYLENE, Cis 1,2- | V | L | 97 | 2.0E+02 | 4.1E-03 | |||||||||||||||||||||||||||||
| DICHLOROETHYLENE, Trans 1,2- | V | L | 97 | 3.3E+02 | 9.3E-03 | |||||||||||||||||||||||||||||
| DICHLOROPROPANE, 1,2- | V | L | 113 | 5.3E+01 | 2.9E-03 | |||||||||||||||||||||||||||||
| DICHLOROPROPENE, 1,3- | V | L | 111 | 3.4E+01 | 3.7E-03 | |||||||||||||||||||||||||||||
| DIOXANE, 1,4- | V | L | 88 | 3.8E+01 | 4.9E-06 | |||||||||||||||||||||||||||||
| ETHANOL | V | L | 46 | 5.9E+01 | 6.3E-06 | |||||||||||||||||||||||||||||
| ETHYLBENZENE | V | L | 106 | 9.6E+00 | 7.8E-03 | |||||||||||||||||||||||||||||
| METHYL ETHYL KETONE | V | L | 72 | 9.1E+01 | 5.6E-05 | |||||||||||||||||||||||||||||
| METHYL ISOBUTYL KETONE | V | L | 100 | 2.0E+01 | 1.4E-04 | |||||||||||||||||||||||||||||
| METHYL TERT BUTYL ETHER | V | L | 88 | 2.5E+02 | 5.9E-04 | |||||||||||||||||||||||||||||
| METHYLENE CHLORIDE | V | L | 85 | 4.4E+02 | 3.2E-03 | |||||||||||||||||||||||||||||
| STYRENE | V | L | 104 | 6.4E+00 | 2.7E-03 | |||||||||||||||||||||||||||||
| tert-BUTYL ALCOHOL | V | L | 74 | 4.1E+01 | 1.2E-05 | |||||||||||||||||||||||||||||
| TETRACHLOROETHANE, 1,1,1,2- | V | L | 168 | 4.6E+00 | 2.4E-03 | |||||||||||||||||||||||||||||
| TETRACHLOROETHANE, 1,1,2,2- | V | L | 168 | 4.6E+00 | 3.7E-04 | |||||||||||||||||||||||||||||
| TETRACHLOROETHYLENE | V | L | 166 | 1.9E+01 | 1.8E-02 | |||||||||||||||||||||||||||||
| TOLUENE | V | L | 92 | 2.8E+01 | 6.6E-03 | |||||||||||||||||||||||||||||
| TPH (gasolines) | V | L | 108 | 6.8E+02 | 7.2E-04 | |||||||||||||||||||||||||||||
| TRICHLOROETHANE, 1,1,1- | V | L | 133 | 1.2E+02 | 1.7E-02 | |||||||||||||||||||||||||||||
| TRICHLOROETHANE, 1,1,2- | V | L | 133 | 2.3E+01 | 8.3E-04 | |||||||||||||||||||||||||||||
| TRICHLOROETHYLENE | V | L | 131 | 6.9E+01 | 9.8E-03 | |||||||||||||||||||||||||||||
| TRICHLOROPROPANE, 1,2,3- | V | L | 147 | 3.7E+00 | 3.4E-04 | |||||||||||||||||||||||||||||
| TRICHLOROPROPENE, 1,2,3- | V | L | 145 | 3.7E+00 | 2.8E-02 | |||||||||||||||||||||||||||||
| VINYL CHLORIDE | V | G | 63 | 3.0E+03 | 2.7E-02 | |||||||||||||||||||||||||||||
| XYLENES | V | L | 106 | 8.0E+00 | 7.1E-03 | |||||||||||||||||||||||||||||
| CHEMICAL PARAMETER | 1Physical State | Molecular Weight | 2Vapor Pressure mm Hg (25C) |
Henry’s Law Constant (H) (atm-m³/mol) |
||||||||||||||||||||||||||||||
| SEMI-VOLATILE AND OTHER SEMI-STABLE CHEMICALS 3,4Subsample Multi Increment Bulk Sample at Laboratory Upon Receipt Without Drying (VP 0.1 to 1.0 OR Liquid at 25C OR Henry’s Constant >1.0E-05) |
||||||||||||||||||||||||||||||||||
| BIPHENYL, 1,1- | *SV | S | 154 | 8.9E-03 | 3.2E-04 | |||||||||||||||||||||||||||||
| BIS(2-CHLOROISOPROPYL)ETHER | *SV | L | 171 | 8.5E-01 | 1.1E-04 | |||||||||||||||||||||||||||||
| DALAPON | *SV | L | 143 | 1.9E-01 | 9.0E-08 | |||||||||||||||||||||||||||||
| DIBROMO,1,2- CHLOROPROPANE,3- | *SV | L | 236 | 5.8E-01 | 1.5E-04 | |||||||||||||||||||||||||||||
| DIMETHYLPHENOL, 2,4- | SV | S | 122 | 1.0E-01 | 9.5E-07 | |||||||||||||||||||||||||||||
| HEXACHLOROBUTADIENE | SV | S | 261 | 2.2E-01 | 1.0E-02 | |||||||||||||||||||||||||||||
| HEXACHLOROETHANE | SV | S | 237 | 4.0E-01 | 3.9E-03 | |||||||||||||||||||||||||||||
| ISOPHORONE | SV | L | 138 | 4.4E-01 | 6.6E-06 | |||||||||||||||||||||||||||||
| 5MERCURY | *SV | S | 201 | 2.0E-03 | – | |||||||||||||||||||||||||||||
| METHYL MERCURY | SV | S | 216 | – | – | |||||||||||||||||||||||||||||
| NITROBENZENE | *SV | L | 123 | 2.5E-01 | 2.4E-05 | |||||||||||||||||||||||||||||
| NITROGLYCERIN | SV | L | 227 | 2.0E-04 | 9.8E-08 | |||||||||||||||||||||||||||||
| NITROTOLUENE, 4- | SV | S | 137 | 1.6E-01 | 5.6E-06 | |||||||||||||||||||||||||||||
| NITROTOLUENE, 2- | *SV | S | 137 | 1.9E-01 | 1.2E-05 | |||||||||||||||||||||||||||||
| NITROTOLUENE, 3- | *SV | S | 137 | 2.1E-01 | 2.4E-05 | |||||||||||||||||||||||||||||
| 6PAHs (varies, see Table 4-2b) | *SV | S | ||||||||||||||||||||||||||||||||
| PHENOL | SV | S | 94 | 3.5E-01 | 3.4E-07 | |||||||||||||||||||||||||||||
| PROPICONAZOLE | SV | L | 342 | 1.0E-06 | 4.1E-09 | |||||||||||||||||||||||||||||
| 7TPH (middle distillates) | *SV | L | 170 | 2 to 26 | 7.2E-04 | |||||||||||||||||||||||||||||
| TRICHLOROBENZENE, 1,2,4- | *SV | S | 181 | 4.6E-01 | 1.4E-03 | |||||||||||||||||||||||||||||
| 3Targeted PAHs | 1Physical State | Molecular Weight | 2Vapor Pressure mm Hg (25C) |
Henry’s Law Constant (H) (atm-m³/mol) |
||||||||||||||||||||||||||||||
| Semi-Volatile PAHs (VP 0.1 to 1.0 OR Liquid at 25C OR Henry’s Constant >1.0E-05) 3,4Subsample Multi Increment Bulk Sample at Laboratory Upon Receipt Without Drying |
||||||||||||||||||||||||||||||||||
| ACENAPHTHENE | *SV | S | 154 | 2.2E-03 | 1.8E-04 | |||||||||||||||||||||||||||||
| ACENAPHTHYLENE | *SV | S | 152 | 6.7E-03 | 1.5E-03 | |||||||||||||||||||||||||||||
| ANTHRACENE | *SV | S | 178 | 6.6E-06 | 5.6E-05 | |||||||||||||||||||||||||||||
| FLUORENE | *SV | S | 166 | 3.2E-04 | 9.5E-05 | |||||||||||||||||||||||||||||
| METHYLNAPHTHALENE, 1- | *SV | S | 142 | 6.7E-02 | 5.1E-04 | |||||||||||||||||||||||||||||
| METHYLNAPHTHALENE, 2- | *SV | S | 142 | 5.5E-02 | 5.1E-04 | |||||||||||||||||||||||||||||
| NAPHTHALENE | *SV | S | 128 | 8.5E-02 | 4.4E-04 | |||||||||||||||||||||||||||||
| PHENANTHRENE | *SV | S | 178 | 1.2E-04 | 3.9E-05 | |||||||||||||||||||||||||||||
| PYRENE | *SV | S | 202 | 4.5E-06 | 1.2E-05 | |||||||||||||||||||||||||||||
| BENZO(a)ANTHRACENE | NV | S | 228 | 5.0E-09 | 1.2E-05 | |||||||||||||||||||||||||||||
| BENZO(a)PYRENE | NV | S | 252 | 5.5E-09 | 4.6E-07 | |||||||||||||||||||||||||||||
| BENZO(b)FLUORANTHENE | NV | S | 252 | 5.0E-07 | 6.6E-07 | |||||||||||||||||||||||||||||
| BENZO(g,h,i)PERYLENE | NV | S | 276 | – | 1.4E-07 | |||||||||||||||||||||||||||||
| BENZO(k)FLUORANTHENE | NV | S | 252 | 9.7E-10 | 5.9E-07 | |||||||||||||||||||||||||||||
| CHRYSENE | NV | S | 228 | 6.2E-09 | 5.1E-06 | |||||||||||||||||||||||||||||
| DIBENZO(a,h)ANTHTRACENE | NV | S | 278 | 9.6E-10 | 1.2E-07 | |||||||||||||||||||||||||||||
| FLUORANTHENE | NV | S | 202 | 9.2E-06 | 8.8E-06 | |||||||||||||||||||||||||||||
| INDENO(1,2,3-cd)PYRENE | NV | S | 276 | 1.2E-10 | 3.4E-07 | |||||||||||||||||||||||||||||
| 1Sample Preparation Method | Subsample Collection Method | |||||||||||||||||||||||||||||||||
| Sectoral Splitter | Manual | |||||||||||||||||||||||||||||||||
| Unground (<2 mm) | 10 g | 30 g | ||||||||||||||||||||||||||||||||
| Ground (<100 µm) | 5 g | 5 g | ||||||||||||||||||||||||||||||||
| Replicate Sample Data Precision | Use of DU Data for Decision Making | |||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Good (RSD ≤ 35%) |
|
|||||||||||||||||||||||||||||||||
| Moderate (35% < RSD ≤ 50%) |
|
|||||||||||||||||||||||||||||||||
| Poor (50% < RSD ≤ 100%) |
|
|||||||||||||||||||||||||||||||||
| Very Poor (RSD > 100%) |
|
|||||||||||||||||||||||||||||||||
| Site name and identifier;
Name(s) of field personnel or sampler; Date, time; Sketch map depicting DU shape, dimensions, adjoining DUs, landmarks, north arrow, etc.; Decision Unit coordinates (e.g., latitude and longitude of DU corners); Photographs of DU areas; Depth of DU sampling interval; |
Increment spacing;
Number of increments collected for bulk Multi Increment sample(s); Type of sampling equipment used; Sample containers; Approximate bulk sample mass; Describe any field processing of sample(s); Soil description; Planned sample analyses; General comments (e.g., odor, staining, etc.). |
|||||||||||||||||||||||||||||||||
| Name of drilling contractor;
Borehole coordinates – latitude and longitude; Sketch showing the borehole location (including reference distances); Diameter and total depth of borehole; Type of drilling equipment used (e.g., direct push, auger, air or mud rotary); Drilling fluid and angle (if applicable); Blow counts/Resistance; Depth to water table |
Important stratigraphic boundaries encountered (e.g. depth bedrock);
Hydrogeologic information (e.g. transmissivity, etc.); General comments (e.g., odor, staining, etc.); Field measurements (e.g., PID, XRF, etc.); Designated DU layers for sample collection; Sample collection equipment used; Core recovery and portion submitted for analysis; Sample identification number; Planned sample analyses |
|||||||||||||||||||||||||||||||||
| Major Divisions | Code | Description | ||||||||||||||||||||||||||||||||
| Coarse-Grained Soils More than 50% retained on a 0.075 mm (No. 200) sieve |
Gravels 50% or more of course fraction retained through a 4.75 mm (No. 4) sieve | Clean Gravels (<5% fines) |
GW | Well-graded gravels, sandy gravels or gravels with sand; little to no fines | ||||||||||||||||||||||||||||||
| GP | Poorly-graded gravels, sandy gravels or gravels with sand; little to no fines | Gravels with Fines (>5% fines) |
GM | Sandy gravels with silt, gravels with sand and silt | GC | Sandy gravels with clay, gravels with sand and clay | Sands 50% or more of course fraction passes through a 4.75 mm (No. 4) sieve | Clean Sands (<5% fines) |
SW | Well-graded sands, gravelly sands, or sands with gravel; little to no fines | SP | Poorly-graded sands, gravelly sands, or sands with gravel; little to no fines | Sands with Fines (>5% fines) |
SM | Silty sands, sands with silt | SC | Clayey sands, sands with clay | Fine-Grained Soils More than 50% passes through a 0.075 mm (No. 200) sieve |
Silts and Clays | ML | Silt, sandy silt, clayey silt, silt with fine sand and clay | CL | Clay, silty clay, clay with silt and fine sand | OL | Organic silt and clay (loam) | Highly Organic Soils | PT | Peat and other highly organic soils | ||||||
| Description | Standard Penetration Test Sampler Field Criteria (N-Value) |
Modified California Sampler Field Criteria (N-Value) |
||||||||||||||||||||||||||||||||
| 1 3/8 in. ID Sampler | 2 in. ID Sampler using 1.43 factor | |||||||||||||||||||||||||||||||||
| Very Loose | 0–4 | 0–6 | ||||||||||||||||||||||||||||||||
| Loose | 4–10 | 6–14 | ||||||||||||||||||||||||||||||||
| Medium Dense | 10–30 | 14–43 | ||||||||||||||||||||||||||||||||
| Dense | 30–50 | 43–71 | ||||||||||||||||||||||||||||||||
| Very Dense | > 50 | > 71 | ||||||||||||||||||||||||||||||||
| Description | Standard Penetration Test Sampler Field Criteria (N-Value) |
Modified California Sampler Field Criteria (N-Value) |
||||||||||||||||||||||||||||||||
| 1 3/8 in. ID Sampler | 2 in. ID Sampler using 1.13 factor | |||||||||||||||||||||||||||||||||
| Very Soft | 0–2 | 0–2 | ||||||||||||||||||||||||||||||||
| Soft | 2-4 | 2-4 | ||||||||||||||||||||||||||||||||
| Medium Stiff | 4-8 | 4-9 | ||||||||||||||||||||||||||||||||
| Stiff | 8-16 | 9-18 | ||||||||||||||||||||||||||||||||
| Very Stiff | 16-32 | 18-36 | ||||||||||||||||||||||||||||||||
| Hard | 32 | 36 | ||||||||||||||||||||||||||||||||