Appendix 21-A
Species Profiles and Exposure/Effects Summary
1. Sea Lettuce (Ulva fasciata)
2. Samoan crab (Scylla serrata)
3. Kona crab (Ranina ranina)
4. White crab (Portunus sanguinolentus)
5. Helmet urchin (Colobocentrotus atratus)
6. Hawaiian limpet (Cellana exarata)
7. Day octopus (Octopus cyanea) and Night Octopus (O. ornatus)
8. Polychaete (Neanthes arenaceodentata)
9. Lobe coral (Porites lobata)
10. Black sea cucumber (Holothuria atra)
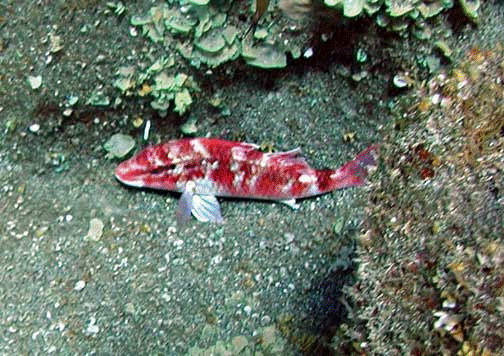
11. Goatfish (Mulloides)
12. Hawaiian flagtail (Kuhlia sandvicensis)
13. Convict tang (Acanthurus triostegus)
14. Pacific sergeant (Abudefduf abdominalis)
15. Mozambique tilapia (Oreochromis mossambicus)
16. Spectacled parrotfish (Chlorurus perspicillatus) and Yellowbar parrotfish (Calotomus zonarchus)
17. Moray eel (Muraenidae)
18. Wedge-tailed shearwater (Puffinus pacificus)
19. Black-crowned night heron (Nycticorax nycticorax hoactli)
20. Hawaiian coot (Fulica alai)
21. Green sea turtle (Chelonia mydas)
22. Monk seal (Monachus schauinslandi)
1 – Sea Lettuce (Ulva fasciata)
 Limu kohu (Asparagopsis taxiformis) |
 Limu pālahalaha (Sea lettuce) (Ulva fasciata) |
Native Hawaiian Species: Numerous native species of seaweed, or limu, occur around the main Hawaiian Islands and the Northwest Hawaiian Islands.
Habitat: Sea lettuce and other limu generally occur in the intertidal zone on rock or coral; a few species grow in sandy locations (Waikīkī Aquarium 2014). Heavy growth may indicate high nutrients and freshwater input from storm water runoff or mouths of streams. Limu is most abundant where wave action is low (University of Hawaiʻi 2001).
Locations: Limu occurs throughout the Hawaiian Islands.
Seasonality (year-round resident or migrant): Year-round resident.
Cultural Use (historical and current): Various species of limu are added to stews and salads; prepared as flavorful condiments; and eaten as a source of vitamins (A, C, B12, and riboflavin) (Preskitt 2002). Some species were used to make traditional hula attire (Waikīkī Aquarium 2014).
Recreational Harvest: Yes, popular for consumption (University of Hawaiʻi 2014, Preskitt 2002).
Commercial Harvest: Yes.
Home Range: Not applicable.
Size/Body Weight: Some species, such as Limu pālahalaha, may grow up to 1 meter in length (University of Hawaiʻi 2001). Tissues of limu may contain up to 86 percent water (McDermid et al. 2007).
Diet/Ingestion Rates: Photosynthetic organisms.
Predators: Consumed by humans, the green sea turtle (McDermid et al. 2007), herbivorous fishes, and sea urchins. Some Hawaiian herbivores appear to prefer to feed on invasive algal species rather than native limu (Vermeij et al. 2009).
Tissue Data (or other use in ecological or human health risk assessment)/Bioaccumulation factors:
- Sea lettuce is high in energy, soluble carbohydrate, protein, vitamin A content, and some minerals (McDermid et al. 2007). In a study of several species of seaweed evaluating their use as bioindicators, sea lettuce had the lowest concentration of metals (El-Din et al. 2014). This study also determined bioconcentration factors for metals in several algal species.
- A total of 19 seaweed samples (species not given) were collected in 2009 from locations on the Waiʻanae Coast of Oʻahu (fall and spring samples), including five samples from a reference location. Samples were analyzed for energetic compounds, metals, phthalate esters, and pyrene (U.S. Army Corps of Engineers [USACE] 2012). Detected constituents at the reference location (Table 9 in USACE2012) included the following:
- Inorganic Constituents: Arsenic, Barium, Chromium, Cobalt, Copper, Lead, Nickel, Strontium, Vanadium, and Zinc
- Organic Constituents: None
- Four seaweed composites samples were collected from the nearshore waters at Mākua (see Figure 2-1 in Tetra Tech 2009). The samples were composites of Acanthophora spicifera, Sargassum muticum, and Sargassum polyphyllum. Samples were analyzed for dioxins/furans, VOCs SVOCs, organochlorine pesticides, explosives, and metals (see Tables 2-2 and 3-1 in Tetra Tech 2009). The sample had 75.4 to 88.4 percent moisture. Detected constituents included the following:
- Inorganic Constituents: Aluminum, Antimony, Arsenic, Barium, Beryllium, Cadmium, Chromium, Cobalt, Copper, Iron, Lead, Manganese, Mercury, Selenium, Silver, Thallium, Vanadium, and Zinc
- Organic Constituents: Dioxins/Furans, m+p-Xylenes, Bis(2-ethylhexyl)phthalate, di-n-Butylphthalate, Aldrin, beta-BHC, Heptachlor, Heptachlor epoxide, Perchlorate, and RDX
Conservation Status: Not threatened.
References
USACE (U.S. Army Corps of Engineers). 2012. Ecological Risk Assessment and HHRA (Appendices to RI): Ordnance Reef (Site HI-06) Waiʻanae, Oʻahu, Hawaiʻi.
El-Din, N.G. Shams, Mohamedein, L.I., and K.M. El-Moselhy. 2014. Seaweeds as bioindicators of heavy metals off a hot spot area on the Egyptian Mediterranean Coast during 2008-2010. Environmental Monitoring and Assessment, 186(9): 5865-5881.
McDermid, K.J., Stuercke, B., and G.H. Balazs. 2007. Nutritional composition of marine plants in the diet of the green sea turtle (Chelonia mydas) in the Hawaiian Islands. Bulletin of Marine Science, 81(1): 55-71.
Preskitt, L. 2002. Edible Limu: Gifts from the Sea. Poster. https://www.hawaii.edu/reefalgae/publications/ediblelimu/index.htm
Tetra Tech, Inc. 2009. Marine Resources Study Field Sampling Results and Risk Assessment, Mākua Military Reservation, Oʻahu, Hawaiʻi.
University of Hawaiʻi. 2001. Algae: Invasive Native, Ulva fasciata. Botany, University of Hawaiʻi at Mānoa.
University of Hawaiʻi, Botany Department. 2014. “ReefWatcher’s Field Guide to Alien and Native Hawaiian Marine Algae.” https://www.hawaii.edu/reefalgae/natives/sgfieldguide.htm
Vermeij, M.J.A., Smith, T.B., Dailer, M.L., and C.M. Smith. 2009. Release from native herbivores facilitates the persistence of invasive marine algae: a biogeographical comparison of the relative contribution of nutrients and herbivory to invasion success. Biol Invasions, 11:1463–1474.
Waikīkī Aquarium. 2014. Limu Pālahalaha. Animal Guide. https://www.waikikiaquarium.org/experience/animal-guide/plants-seaweeds/seaweeds/limu-palahalaha/ Accessed September 2, 2014. Updated URL: https://www.waikikiaquarium.org/experience/plants-seaweeds/seaweeds/limu-palahalaha/.
Photo Credit: Preskitt, L. 2002. Edible Limu: Gifts from the Sea. Poster. https://www.hawaii.edu/reefalgae/publications/ediblelimu/index.htm
2 – Samoan crab (Scylla serrata)
 Samoan crab (Scylla serrata) |
Native Hawaiian Species: No. Crabs from Sāmoa were released on Oʻahu, Molokaʻi, and Hawaiʻi to establish a commercial crab fishery (Eldredge and Smith 2001).
Habitat: This crab inhabits muddy bottoms in brackish water along the shoreline, mangrove areas, and river mouths (Eldredge and Smith 2001). During the day, it may live intertidally in burrows but mostly buries in the mud at subtidal levels (Rowling and Ives 2010).
Locations: All main islands (Eldredge and Smith 2001).
Cultural Use (historical and current): None found.
Recreational Harvest: Yes, but no harvest numbers available.
Commercial Harvest: Prized, sought-after commercial species (Eldredge and Smith 2001).
Home Range: In Australia, apart from spawning migrations, the mud crab appears to move little within its habitat; most individuals remain on site in distinct populations (Shelley and Lovatelli 2011). However, longer-term tagging has shown that individuals can move several kilometers from their home range over time; nightly movements of S. serrata fitted with transmitters averaged 461 meters (Shelley and Lovatelli 2011).
Size/Body Weight: It is the largest portunid in Hawaiʻi, exceeding 18 cm in width of carapace (Eldredge and Smith 2001). It can reach 28 cm in carapace width and 3 kg in weight but is more commonly 15 to 20 cm in width and 0.5 to 1.0 kg (Rowling and Ives 2010).
Diet/Ingestion Rates: It is primarily a carnivore, eating mollusks, crustaceans, and polychaetes, as well as small amounts of plants and debris (Eldredge and Smith 2001). Feeding rates are linked to body weight. On a wet weight basis, feeding rates were reported as 5 to 10 percent of body weight (Baliao, De Los Santos & Franco 1999; Quinitio 2004 [as cited in (Rowling and Ives 2010)]). No food or sediment ingestion rates were found.
Predators: No predators other than humans were reported.
Tissue Data (or other use in ecological or human health risk assessment)/Bioaccumulation Factors: One sample of Samoan crab from the Mākua north muliwai was analyzed for dioxins/furans, VOCs SVOCs, organochlorine pesticides, explosives, and metals (see Figure 2-1 and Tables 2-2 and 3-1 in Tetra Tech 2009). The sample had 0.7 percent lipids and 71.3 percent moisture. Detected constituents included the following:
- Inorganic Constituents: Aluminum, Arsenic, Barium, Chromium, Cobalt, Copper, Iron, Manganese, Mercury, Selenium, Vanadium, and Zinc
- Organic Constituents: Dioxins/furans
Conservation Status: No special status, but taking females is prohibited (Hawaiʻi DLNR Fishing Regulations).
Other Notes:
The Samoan crab was first introduced into Kāneʻohe Bay to start a fishery in 1926 (Eldredge and Smith 2001). Between 1926 and 1935, 98 crabs were released on Oʻahu, Hawaiʻi, and Molokaʻi, all from Sāmoa (Brock 1960, as cited in Eldredge and Smith 2001). By 1940 it had “already become thoroughly established about the shores, entering estuaries of streams and ascending far up some of the larger rivers” (Edmondson and Wilson 1940, as cited in Eldredge and Smith 2001).
References
Eldredge, L.G. & Smith, C.M. Eds. 2001. A Guidebook of Introduced Marine Species in Hawaiʻi. Bishop Museum Technical Report 21, August. https://www2.bishopmuseum.org/hbs/invertguide/download.htm
Hawaiʻi DLNR Fishing Regulations. 2014. https://dlnr.hawaii.gov/dar/fishing/fishing-regulations/marine-invertebrates/ Accessed August 2014.
Rowling, K., Hegarty, A., Ives, M. 2010. Status of Fisheries Resources in NWS 2008/09. Industry & Investment NSW, Cronilla. 392 pp. https://www.dpi.nsw.gov.au/research/areas/fisheries-and-ecosystems/wild-fisheries/outputs/2010/1797/StatusOfFisheriesResourcesNSW2008-09.pdf
Shelley, C., Lovatelli, A. 2011. Mud crab aquaculture – A practical manual FAO Fisheries and Aquaculture Technical Paper. No. 567. Rome, FAO. 78 pp. https://www.fao.org/docrep/015/ba0110e/ba0110e.pdf
Tetra Tech, Inc. 2009. Marine Resources Study Field Sampling Results and Risk Assessment, Mākua Military Reservation, Oʻahu, Hawaiʻi.
Photo Credit: Eldredge and Smith. 2001
3 – Kona crab (Ranina ranina)
 Kona crab (Ranina ranina) |
Native Hawaiian Species: Yes
Habitat: The Kona crab is found in offshore coastal environmental at depths between 6 and 200 meters. It prefers sandy ocean bottoms where it burrows in the sand (Fielding and Haley 1976). It spends 95% of its time buried in sand and emerges from the sand less than two hours a day, on average (Skinner and Hill 1986).
Locations: Found throughout the Indo-Pacific region and in the Hawaiian Islands (Fielding and Haley 1976).
Seasonality (year-round resident or migrant): Year-round resident.
Cultural Use (historical and current): None identified.
Recreational Harvest: Yes. Spearing these crabs is not permitted (Hawaiʻi Administrative Rules, 1989).
Commercial Harvest: Yes, this species is harvested commercially in Hawaiʻi (Fielding and Haley 1976). Per Hawaiian fishing regulations, the minimum size to harvest is 4 inches (carapace length) (Hawaiʻi Administrative Rules, 1989). Females cannot be collected, per a 2006 ruling.
Home Range: Not reported.
Size/Body Weight: Mature female Kona crabs have a minimum carapace length of 86 ± 8 mm (Fielding and Haley 1976). The estimated time for a Kona crab to reach 100 mm in length ranged from approximately 6 years for females to 4 years for males (Kirkwood et al. 2005). The estimated mean maximum lengths of the Kona crab is 122 mm for females and 156 mm for males, based on commercial catch data (Kirkwood et al. 2005).
Diet/Ingestion Rates: This crab is likely a scavenger, given the composition of items found in its gut (Baylon and Tito 2012, Skinner and Hill 1986). Kona crabs in the Philippines feed on fish (Sardinella), other crabs, shrimp, bivalves, rays, hydroids, copepods, and squid (Baylon and Tito 2012). Silt and sand accounted for 12 to 20 percent of gut contents (Baylon and Tito 2012). In Australia, echinoderms were the most common item in the gut of Kona crabs, followed by polychaetes and fish (Skinner and Hill 1986).
Predators: Humans, sharks, rays, jacks, turtles, and marine mammals (Thomas, undated).
Tissue Data (or other use in ecological or human health risk assessment)/Bioaccumulation Factors:
- 28 crabs (species inferred to be Kona crab) collected in 2009 from three locations on the Waiʻanae Coast of Oʻahu (fall and spring samples) were analyzed for energetic compounds, metals, phthalate esters, and pyrene (U.S. Army Corps of Engineers [USACE] 2012). Detected constituents (as shown in Table 8 in USACE 2012) included the following:
- Inorganic Constituents: Arsenic, Barium, Cadmium, Chromium, Cobalt, Copper, Lead, Mercury, Selenium, Strontium, and Zinc
- Organic Constituents: 1,3,5-Trinitrobenzene.
- One Kona crab sample was collected from nearshore waters at Mākua and analyzed for dioxins/furans, VOCs, SVOCs, organochlorine pesticides, explosives, and metals (see Figure 2-1 and Tables 2-2 and 3-1 in Tetra Tech 2009). The sample had 21 percent lipids and 61.5 percent moisture. Detected constituents included the following:
- Inorganic Constituents: Aluminum, Arsenic, Barium, Cadmium, Chromium, Cobalt, Copper, Iron, Lead, Manganese, Selenium, Vanadium, and Zinc
- Organic Constituents: None
Conservation Status: Not threatened.
References
USACE (U.S. Army Corps of Engineers), 2012. Ecological Risk Assessment and HHRA (Appendices to RI): Ordnance Reef (Site HI-06) Waiʻanae, Oʻahu, Hawaiʻi.
Baylon, J.C. and O.D. Tito. 2012. Natural Diet and Feeding Habits of the Red Frog Crab (Ranina ranina) from Southwestern Mindanao, Philippines. Philipp Agric Scientist, 95(4): 391–398.
Fielding, A. and S.R. Haley. 1976. Sex Ratio, Size at Reproductive Maturity, and Reproduction of the Hawaiian Kona Crab, Ranina ranina (Linnaeus) (Brachyura, Gymnopleura, Raninidae). Pacific Science, 30(2): 131-145.
Hawaiʻi Administrative Rules. 1989. Rules Regulating the Taking and Selling of Certain Marine Resources. Title 13. Chapter 95. Effective 12/03/1998. Revised 12/19/2002. https://dlnr.hawaii.gov/dar/files/2014/05/ch95.pdf
Kirkwood, J.M., Brown, I.W., Gaddes, S.W. and S. Hoyle. 2005. Juvenile length-at-age data reveal that spanner crabs (Ranina ranina) grow slowly. Marine Biology, 147: 331–339.
Skinner, D.G. and Hill, B.J. 1986. Catch rate and emergence of male and female spanner crabs (Ranina ranina) in Australia. Marine Biology, 91: 461-465.
Tetra Tech, Inc. 2009. Marine Resources Study Field Sampling Results and Risk Assessment, Mākua Military Reservation, Oʻahu, Hawaiʻi.
Thomas, L. (Undated). Characterizing the Kona crab (Ranina ranina) fishery in the Main Hawaiian Islands. Hawaiʻi Pacific University. Presentation. https://www.soest.hawaii.edu/PFRP/dec10mtg/thomas_kahng.pdf
Photo Credit: “Ranina ranina ” by Kzhr – Kzhr’s file. Licensed under Creative Commons Attribution-Share Alike 2.5 via Wikimedia Commons. https://commons.wikimedia.org/wiki/File:Ranina_ranina.jpg#mediaviewer
4 – White crab (Portunus sanguinolentus)
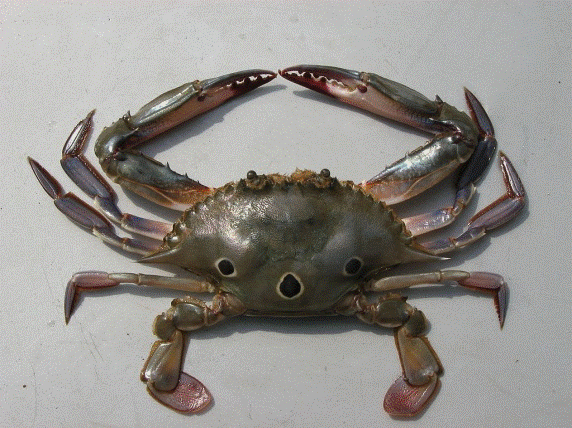 White crab (Portunus sanguinolentus) |
Native Hawaiian Species: Yes. The white crab is also known as the three-spot swimming crab (Atlas of Living Australia 2014).
Habitat: The white crab is found on sandy ocean floors at depths around 30 meters (Sumpton et al. 1989, Carpenter et al. 1997 [as cited in Rasheed and Mustaquim 2010]). It is also reported to inhabit sandy and muddy substrates in shallow coastal waters from 10-30 meters but can occur at depths outside coastal waters reaching 80 meters (Atlas of Living Australia 2014).
Locations: This crab is found throughout the Indo-Pacific region and in the Hawaiian Islands (Apel and Spiridonov 1998 [as cited in Rasheed and Mustaquim 2010]).
Seasonality (year-round resident or migrant): Year-round resident.
Cultural Use (historical and current): None identified.
Recreational Harvest: Yes
Commercial Harvest: Yes
Home Range: A related species of crab (Portunus pelagicus) in the coastal water of the South China Sea was reported to travel mean distances of 7.36 km ± 1.78 (males) and 9.15 km ± 1.87 (females) (Ikhwanuddin et al. 2012). The movement of the crabs was attributed to migration associated with reproduction as the male crabs moved to deeper off-shore areas and the female crabs moved both to deeper off-shore and shallow near-shore areas. Generally, crabs were recaptured within a 2-km radius of the sampling site (Ikhwanuddin et al. 2012). A study of Portunus pelagicus in Australia reported similar movements, with 79% caught within 2 km of the release point and 4% caught more than 10 km from the release point (Potter et al. 1991 [as cited in Ikhwanuddin et al. 2012]).
Size/Body Weight: The maximum sizes of white crabs captured in one study were 125 mm short carapace width (SCW) for males (n = 233) and 130 mm SCW for females (n = 224) (Rasheed and Mustaquim 2010). Juvenile and adult crabs were captured. The study determined that the crabs were mature at 64–69 mm SCW or 83–89mm long carapace width (LCW) for males and 63–71mm SCW or 81–93 mm LCW for females (Rasheed and Mustaquim 2010). An investigation of the white crab in Australia determined mature males were 83 mm long carapace width (LCW) and mature females were 74 mm LCW (Sumpton et al. 1989 [as cited in Rasheed and Mustaquim 2010]).
Diet/Ingestion Rates: The white crab eats mostly crustaceans (47%) followed by fish (29%) and mollusks (6%); sand; it also consumes sand/mud/debris (5%) (Sukumaran and Neelakantan 1997). It is reported to scavenge dead fish discarded by fishing vessels (Paul 1981, and Wasseflberg and Hill 1982 [as cited in Sukumaran and Neelakantan 1997]). It also feeds on detritus (Atlas of Living Australia 2014).
Predators: Predators include turtles, sharks, rays and large fish.
Tissue Data (or other use in ecological or human health risk assessment)/Bioaccumulation Factors:
Conservation Status: Not threatened.
References
Atlas of Living Australia, 2014. https://bie.ala.org.au/species/PORTUNUS%20SANGUINOLENTUS Accessed August 2014. Updated URL: https://bie.ala.org.au/species/urn:lsid:biodiversity.org.au:afd.taxon:7a846ec4-d262-4853-b26c-9e80cc73603e
Ikhwanuddin, M., Nurfaseha, A.H., Abol-Munafi, A.B. And M.L. Shabdin, 2012. Movement Patterns of Blue Swimming Crab, Portunus Pelagicus in the Sarawak Coastal Water, South China Sea. Journal of Sustainability Science and Management, 7(1): 1-8.
Rasheed, S. and J. Mustaquim, 2010. Size at sexual maturity, breeding season and fecundity of three-spot swimming crab Portunus sanguinolentus (Herbst, 1783) (Decapoda, Brachyura, Portunidae) occurring in the coastal waters of Karachi, Pakistan. Fisheries Research, 103: 56–62.
Sukumaran, K.K. and B. Neelakantan, 1997. Food and feeding of Portunus (Portunus) sanguinolentus (Herbst) and Portunus (Portunus) pelagicus (Linnaeus) (Brachyura: Portunidae) along Karnataka coast. Indian Journal of Marine Sciences, 26: 35-38.
Photo Credit: Portunus sanguinolentus by Self, Licensed under GNU Free Documentation License via https://commons.wikimedia.org/wiki/File:Portunus_sanguinolentus.jpg Updated URL: https://commons.wikimedia.org/wiki/Category:Portunus_sanguinolentus
5 – Helmet urchin (Colobocentrotus atratus)
 Helmet urchin (Colobocentrotus atratus) |
Native Hawaiian Species: Yes. Also known as the shingle urchin or hāʻukeʻuke kaupali (Parrish et al. 1990).
Habitat: The helmet urchin is abundant in the intertidal zone, often on vertical substrates and in shallow basalt pavement habitats (Parrish et al. 1990). The helmet urchin inhabits areas of high wave energy (Waikīkī Aquarium 2014).
Locations: Indo-Pacific region and throughout the Hawaiian Islands (Waikīkī Aquarium 2014).
Seasonality (year-round resident or migrant): Year-round resident.
Cultural Use (historical and current): Historically, other sea urchins (Echinothrix calamaris and Echinothrix diadema) were preferred for food over the helmet urchin (Parrish et al. 1990).
Recreational Harvest: The helmet urchin is harvested for food, particularly the eggs (Parrish et al. 1990).
Commercial Harvest: Yes.
Home Range: Not identified.
Size/Body Weight: The helmet urchin may reach 3 inches in diameter (Waikīkī Aquarium 2014).
Diet/Ingestion Rates: Feeds on algae (Parrish et al. 1990). The helmet urchin grazes predominately on red coralline algae (Waikīkī Aquarium 2014).
Predators: Predators of sea urchins in general include humans and large fish (such as Balistidae, Labridae, Lethrinidae, Gaterinidae and Lutjanidae (McClanahan 1998).
Tissue Data (or other use in ecological or human health risk assessment)/Bioaccumulation Factors:
- The helmet urchin (Colobocentrotus atratus) was collected from tidal pools at ʻĪlio Point, Molokaʻi in 2010 from locations near a debris pile site and a reference location 150 meters northeast of the debris pile site (see Figure 9 in ESI 2012). Individuals weighed 41 to 87 grams and were 3.8 to 7.5 cm in length (see Table 2-12 in ESI 2012). Tissue samples were analyzed for metals and PCBs (see Tables 2-18 and 2-19 in ESI 2012). No significant difference was noted between site and reference samples.
- Two composite samples were collected from nearshore waters at Mākua and three composite samples from a background location at Sandy Beach (see Figure 2-1 and Subsection 5.4.1 in Tetra Tech 2009). A single sample required collecting more than 100 sea urchins (see Subsection 3.1 in Tetra Tech 2009). Samples were analyzed for dioxins/furans, VOCs, SVOCs, organochlorine pesticides, explosives, and metals (see Tables 2-2 and 3-1 in Tetra Tech 2009). Samples ranged from 0.77 to 2.7 percent lipids and 37.7 to 48.1 percent moisture. Detected constituents included the following:
- Inorganic Constituents: Aluminum, Arsenic, Barium, Beryllium, Chromium, Cobalt, Copper, Iron, Lead, Manganese, Selenium, Vanadium, and Zinc
- Organic Constituents: Dioxins/Furans, Toluene, Aldrin, and Perchlorate
- Sediment pore water from Hanalei Bay on the north coast of Kauaʻi and a reference location at Kēʻē Beach, Kauaʻi, were tested for effects on fertilization and embryonic development of the purple-spined sea urchin (Arbacia punctulata), which is generally used as a surrogate echinoderm for toxicity testing of sediment pore water (Carr et al. 2006). Toxicity was reported at two stations; however, no synoptic chemical analyses were conducted to identify the cause of the toxicity.
- Sea urchin fertilization tests and larval development tests were used to evaluate toxicity of sediment pore water in Australia (McCready et al. 2006).
Conservation Status: Not threatened.
Other Notes: Sea urchins may have a beneficial effect on coastal ecosystems through foraging on invasive seaweeds. Native Hawaiian collector urchins (Tripneustes gratilla) were released in Kāneʻohe Bay to control fast growing seaweed on coral reefs (Department of Land and Natural Resources 2014).
References
Carr, R.S., Nipper, M., Field, M., and J.M. Biedenbach, 2006. Coastal Circulation and Sediment Dynamics in Hanalei Bay, Kauaʻi. Part III: Studies of Sediment Toxicity.U.S. Geological Survey Open-File Report 2006-1147.
Department of Land and Natural Resources, 2014. 200,000th Collector Sea Urchin Planted to Protect Kāneʻohe Bay Native Hawaiian Species Protects Coral Reefs from Invasive Seaweeds. Aquatic Resource News Release. March 28, 2014. https://dlnr.hawaii.gov/blog/2014/03/28/nr14-039/
ESI (Environmental Science International), 2012. Removal Action Report, Former U.S. Coast Guard Long Range Navigation (LORAN) Station, ʻĪlio Point, Molokaʻi, Hawaiʻi. July.
McClanahan, T.R. (1998). “Predation and the distribution and abundance of tropical sea urchin populations.” Journal of Experimental Marine Biology and Ecology 221(2): 231-255.
McCready, S., Birch, G.F., Long, E.R., Spyrakis, G., and C.R. Greely, 2006. An Evaluation of Australian Sediment Quality Guidelines. Arch. Environ. Contam. Toxicol. 50: 306–315.
Parrish, J., G. Smith, and J. Norris. 1990. Resources of the marine waters of Kaloko-Honokōhau National Historical Park. Cooperative National Park Resources Study Unit, Technical Report 74. Department of Botany, University of Hawaiʻi. Honolulu. 118 pp. https://manoa.hawaii.edu/hpicesu/techr/074.pdf Updated URL: https://scholarspace.manoa.hawaii.edu/handle/10125/5886
Tetra Tech, Inc. 2009. Marine Resources Study Field Sampling Results and Risk Assessment, Mākua Military Reservation, Oʻahu, Hawaiʻi.
Waikīkī Aquarium, 2014. Shingle Urchin. Animal Guide. https://www.waikikiaquarium.org/experience/animal-guide/invertebrates/echinoderms/shingle-urchin/ Accessed August 28, 2014.
Photo Credit: “Colobocentrotus atratus. Hāʻukeʻuke kaupali. Helmet urchin” Photo taken by Bryan Harry, National Park Service. https://www.botany.hawaii.edu/basch/uhnpscesu/htms/kahoinvr/fish_pops/echinomet/wana05.htm
6 – Hawaiian limpet (Cellana exarata)
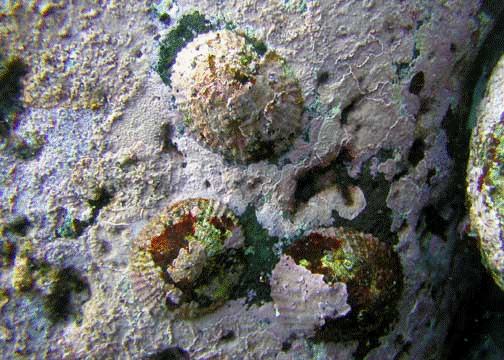 Hawaiian limpet (Cellana exarata) ʻopihi |
Native Hawaiian Species: Yes. Known locally as ʻopihi. Three species are endemic to the Hawaiian Islands: blackfoot (Cellana exarata), yellowfoot (Cellana sandwicensis), and giant ʻopihi (Cellana talcosa) (Bird et al. 2007).
Habitat: The three endemic Hawaiian limpets inhabit different positions on wave-exposed rocky shores. C. exarata inhabits the high intertidal zone, followed by C. sandwicensis at the low intertidal zone, and C. talcosa in the shallow subtidal zone (Kay and Magruder 1977, C.E.B. unpublished data [as cited in Bird et al. 2007]). C. talcosa is submerged at high tide but occurs no deeper than 3 to 4 meters (Bird 2011).
Locations: Throughout the MHI and NWHI (Bird et al. 2007).
Seasonality (year-round resident or migrant): Year-round resident.
Cultural Use (historical and current): Hawaiian limpets are a component of culinary culture (Bird et al. 2007).
Recreational Harvest: Yes. All species of Cellana must be 31.8 mm shell length to harvest (Hawaiʻi Administrative Rules 1981).
Commercial Harvest: Yes.
Home Range: Not identified; but assumed small.
Size/Body Weight: Mature C. sandwicensis have a shell length of 20 mm and mature C. talcosa have a shell length of 35 to 40 mm (Kay et al. 2006).
Diet/Ingestion Rates: Algae.
Predators: Humans, intertidal thaid gastropods, and crabs. C. talcosa is also exposed to predatory fish (Bird 2011).
Tissue Data (or other use in ecological or human health risk assessment)/Bioaccumulation Factors: The Hawaiian limpet (Cellana exarata) was collected from tide pools at ʻĪlio Point, Molokaʻi in 2010 from locations near a debris pile site and a reference location 150 meters northeast of the debris pile site (see Figure 9 in ESI 2012). Individuals weighed 10 to 40 grams and were 3.1 to 5.5 cm in length (see Table 2-12 in ESI 2012). Samples were analyzed for metals and PCBs (see Tables 2-18 and 2-19 in ESI 2012). No significant difference was noted between results for site and reference samples.
Conservation Status: Not threatened.
References
Bird, C.E. 2011. Morphological and Behavioral Evidence for Adaptive Diversification of Sympatric Hawaiian Limpets (Cellana spp.). Integrative and Comparative Biology, 51 (3): 466–473.
Bird, C.E., Holland, B.S., Bowen, B.W., and R.J. Toonen, 2007. Contrasting phylogeography in three endemic Hawaiian limpets (Cellana spp.) with similar life histories. Molecular Ecology, 16: 3173–3186.
ESI (Environmental Science International), 2012. Removal Action Report. Former U.S. Coast Guard Long Range Navigation (LORAN) Station, ʻĪlio Point, Molokaʻi, Hawaiʻi. July.
Hawaiʻi Administrative Rules, 1981. ʻOpihi. Title 13. Chapter 92. Effective 8/4/1987. Revised 5/26/1981.
Kay, E.A., Bird, C.E., Holland B.S., and C.M. Smith, 2006. Growth Rates, Reproductive Cycles, and Population Genetics of ʻOpihi from the National Parks in the Hawaiian Islands. In Brown et al., 2006. Ecology of KALA Marine Resources. https://www.botany.hawaii.edu/basch/uhnpscesu/pdfs/KALAkay06.pdf
Photo Credit: “Cellana sandwicensis. ʻopihi ʻalinalina. yellow-foot ʻopihi” Photo taken by Larry Basch, National Park Service. https://www.botany.hawaii.edu/basch/uhnpscesu/htms/kahoinvr/fish_pops/patell/shell02.htm
7 – Day octopus (Octopus cyanea) and Night Octopus (O. ornatus)
 Day Octopus and Night Octopus (Octopus cyanea) and (Octopus ornatus) heʻe and heʻe-mākoko |
Native Hawaiian Species: Yes. The day octopus (Octopus cyanea) and the night octopus (Octopus ornatus) are common in Hawaiʻi (Waikīkī Aquarium 2014).
Habitat: The day octopus is a common Pacific coral reef resident. It may excavate holes or occupy existing crevices in rocky areas from the intertidal zone to depths of about 45 meters on reef flats and reef slopes (Van Heukelem 1976; Sims 1998). The day octopus in Kāneʻohe Bay most often dens in areas of loose rocks and broken coral on a sandy bottom (Sims 1998). An octopus den is not permanent but may be occupied for several days (Forsythe and Halon 1997). Once inside the den, the octopus may pull loose rocks or rubble over the opening, camouflaging the den and its entrance (Forsythe and Halon 1997).
Locations: Throughout the Hawaiian Islands (Waikīkī Aquarium 2014)
Seasonality (year-round resident or migrant): Year-round resident
Cultural Use (historical and current): None identified
Recreational Harvest: Yes. The day octopus is often collected with a three-pronged spear in shallow depths or by handline (Monterey Bay Aquarium Seafood Watch 2014).
Commercial Harvest: Yes.
In terms of biomass caught, heʻe ranked 28th of the 87 species/categories commercially fished in Hawaiʻi in 2011 at 35,347 pounds and had the highest catch of any invertebrate fishery. Combined with catch from the recreational fishery, the overall heʻe catch is likely to be at least twice as large. O. cyanea comprised approximately 45% and 26,000 pounds of the estimated total annual harvest (1991) of fishes and invertebrate species in Kāneʻohe Bay (Everson 1994 [as cited in Sims, 1998]).
Home Range: Distances traveled during hunting trips for prey ranged from 3 to 91 meters in six individual octopus studied within constructed ponds. The maximum distances ranged from 21 to 91 meters (Yarnall 1969). The estimated total forage distance observed in two octopuses on a coral atoll in French Polynesia was 15 to 120 meters with average distances for each octopus of 52 ± 9.3 meters and 65 ± 12.7 meters (Forsythe and Hanlon 1997). The octopus foraged both morning and afternoon, travelling more than 100 meters a day for food (Forsythe and Hanlon 1997).
Size/Body Weight: The day octopus has been reported to reach 6 kilograms (Van Heukelem 1983; Roper and Hochberg 1988 [as cited in Forsythe and Hanlon 1997]), although typical specimens of both species do not exceed 4.5 kilograms (Waikīkī Aquarium 2014).
Diet/Ingestion Rates: The diet of the day octopus in Kāneʻohe Bay, Oʻahu, is dominated by five genera of crabs, including Thalamita and Leptodius (Mather et al. 2012). Bivalve and gastropod mollusks are also eaten (Forsythe and Hanlon 1997).
Predators: Humans, Hawaiian monk seal, eels, large fish
Tissue Data (or other use in ecological or human health risk assessment)/Bioaccumulation Factors:
- Pearl Harbor Ecological Risk Assessment: Octopus tissues were analyzed for 16 metals and 19 energetic compounds at four marine areas in spring and fall: (1) discarded military munitions (ordnance) locations; (2) the Waiʻanae wastewater treatment plant outfall area; (3) the coastal non-point source discharge area; and (4) a control area with habitat similar to the ordnance area but assumed not to contain discarded munitions. Metals concentrations in octopus tissue were generally higher in samples collected in spring than in fall (Navy 2007b).
- A total of 36 whole-body samples of octopus were collected in 2009 from locations on the Waiʻanae Coast of Oʻahu (fall and spring samples) including eight samples from a reference location. Samples were analyzed for energetic compounds, metals, phthalate esters, and pyrene (U.S. Army Corps of Engineers [USACE] 2012). Metals concentrations in octopus tissue were higher in the spring than the fall (this is opposite of the pattern observed in goatfish also sampled in this investigation). Detected constituents at the reference location as shown in Table 7 (USACE 2012) included the following:
- Inorganic Constituents: Arsenic, Barium, Cadmium, Chromium, Cobalt, Copper, Lead, Mercury, Selenium, Strontium, and Zinc
- Organic Constituents: None
Conservation Status: Not threatened.
Other Notes:
The day octopus lives about 12 to 15 months (Van Heukelem 1976 [as cited in Sims 1998). It mates once, then dies soon after (Sims 1998). Upon reaching sexual maturity, an unmated female lays a clutch of unfertilized eggs, then dies (Sims 1998).
References
USACE (U.S. Army Corps of Engineers). 2012. Ecological Risk Assessment and HHRA (Appendices to RI): Ordnance Reef (Site HI-06) Waiʻanae, Oʻahu, Hawaiʻi.
Forsythe, J.W. and R.T. Hanlon, 1997. Foraging and associated behavior by Octopus cyanea Gray, 1849 on a coral atoll, French Polynesia. Journal of Experimental Marine Biology and Ecology 209: 15-31.
Mather, J.A., Leite, T.S., and A.T. Batista, 2012. Individual prey choices of octopuses: Are they generalist or specialist? Current Zoology 58(4): 597–603.
Monterey Bay Aquarium Seafood Watch. 2014. Day Octopus and Night Octopus: Octopus cyanea and Octopus ornatus. https://www.seachoice.org/wp-content/uploads/2014/03/MBA_SeafoodWatch_HIOctopusReport.pdf
Navy. 2007. Step 7 Baseline ERA, Pearl Harbor: Sediment Remedial Investigation.
Sims, M.A. Population Density of Octopus cyanea in Kāneʻohe Bay. Sea Grant/DLNR/Aquasearch Undergraduate Research Fellowship Program. https://scholarspace.manoa.hawaii.edu/handle/10125/23456
Van Heukelem, W.F. 1976. Growth, bioenergetics, and Life-Span of Octopus cyanea and Octopus maya . Ph.D. Dissertation, University of Hawaiʻi. 232 pages.
Waikīkī Aquarium, 2014. Octopus. Animal Guide. https://www.waikikiaquarium.org/experience/animal-guide/invertebrates/molluscs/octopus/ Accessed August 27, 2014.
Yarnall, J.L., 1969. Aspects of the Behaviour of Octopus Cyanea Gray. Animal Behaviour 1969, 17: 747-724.
Photo Credit: “Octopus ornatus” Dr. Dwayne Meadows, NOAA/NMFS/OPR https://www.photolib.noaa.gov/index.html Updated URL: https://photolib.noaa.gov/Collections/Coral-Kingdom/Other/emodule/752/eitem/31513
8 – Polychaete (Neanthes arenaceodentata)
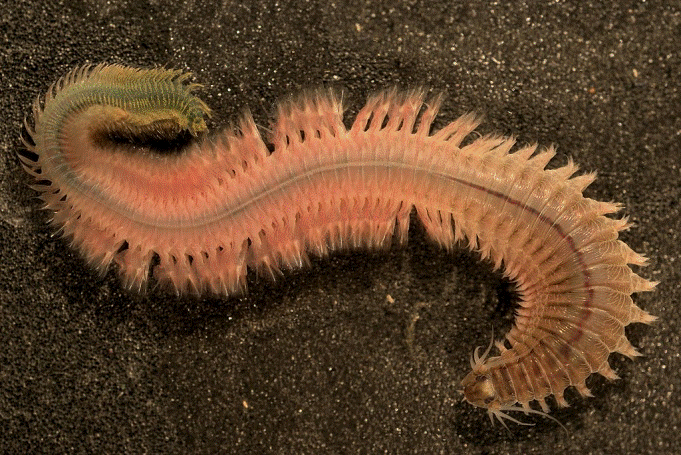 Polychaete (Neanthes arenaceodentata) |
Native Hawaiian Species: not determined.
Habitat: This polychaete occurs in sandy sediments near ocean outfalls (Bailey-Brock et al. 2002). Neanthes arenaceodentata co-occurs with Neanthes succinea in a variety of marine and estuarine intertidal to subtidal habitats, including sand and mud bottoms, seagrass meadows, rocky benthic areas, mussel and oyster beds, and dock pilings (Orth 1973, Craig et al. 2003 [as cited in Masterson 2008]). This worm generally occurs from 10 to 15 cm beneath the sediment surface (Hines and Comtois 1985 [as cited in Masterson 2008]).
Locations: Throughout the Hawaiian Islands.
Seasonality (year-round resident or migrant): Year-round.
Cultural Use (historical and current): None identified.
Recreational Harvest: None.
Commercial Harvest: None.
Home Range: Not reported but assumed small based on observed behavior.
Size/Body Weight: Approximately 5 to 12 mm in length (Bailey-Brock et al. 2002).
Diet/Ingestion Rates: Omnivorous, feeding on particles and debris of various sizes (Bailey-Brock et al. 2002).
Predators: Numerous birds and fish feed extensively on benthic invertebrates, including polychaetes.
Tissue Data (or other use in ecological or human health risk assessment)/Bioaccumulation Factors: Neanthes arenaceodentata is commonly used in laboratory tests. Standardized laboratory toxicity tests have focused predominantly on bioaccumulation in N. arenaceodentata, which is known to for its high sediment ingestion rates and rapid bioaccumulation of organic compounds such as PCBs (Janssen et al. 2011). A new 96-hour feeding test using N. arenaceodentata is being developed to evaluate sublethal effects following exposure to toxic water or sediment (Burton et al. 2011).
Conservation Status: Not threatened.
Other notes: Neanthes arenaceodentata is recognized internationally as a bioindicator for contaminated sediments.
References
Bailey-Brock, J.H., Paavo, B., Barrett, B.M., and J. Dreyer, 2002. Polychaetes Associated with a Tropical Ocean Outfall: Synthesis of a Biomonitoring Program off Oʻahu, Hawaiʻi. Pacific Science, 56 (4): 459-479.
Burton, G.A., B. Chadwick, G. Rosen, and M. Greenberg. 2011. Ecosystem Assessment Protocol (SEAP): An Accurate and Integrated Weight-of-Evidence Based System. SERDP Project ER-1550. 162 pages. January.
Janssen, E.M.L., J.K. Thompson, et al. 2011. PCB-Induces Changes of a Benthic Community and Expected Ecosystem Recovery Following in site Sorbent Amendment. Environmental Toxicology and Chemistry, 30(8): 1819-1826.
Masterson, 2008. Neanthes succinea. Smithsonian Marine Station at Fort Pierce. Expanded Species Reports. https://www.sms.si.edu/irlspec/Neanthes_succinea.htm
Photo Credit: “Alitta succinea” by Hans Hillewaert Licensed under Public domain via Wikimedia Commons https://commons.wikimedia.org/wiki/Alitta_succinea#mediaviewer/File:Alitta_succinea_2.jpg Updated URL: https://commons.wikimedia.org/wiki/Category:Alitta_succinea
9 – Lobe coral (Porites lobata)
 Lobe coral (Porites lobata) |
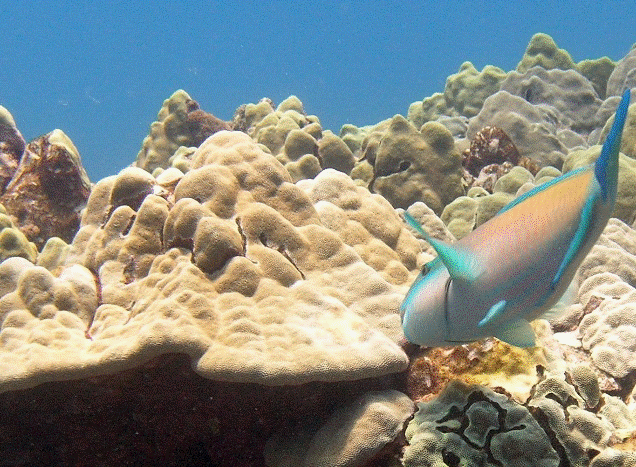 Lobe coral (Porites lobata) |
Native Hawaiian Species: This is one of several species of stony coral native to Hawaiʻi.
Habitat: This reef building coral is dominant all habitats, including forereef, backreef, and lagoons (Kenyon et al. 2006). Porites occurs at depths up to 30 meters (Sheppard et al. 2014).
Locations: Porites lobata is the most abundant coral species throughout the Hawaiian Islands.
Seasonality (year-round resident or migrant): Year-round resident.
Cultural Use (historical and current): Historically, coral reef ecosystems have provided food and medicine (Hawaiʻi Coral Reef Initiative 2002).
Recreational Harvest: None.
Commercial Harvest: None. Commercial harvesting of live coral is illegal within Hawaiian waters (Friedlander et al. 2008a,b).
Home Range: Not applicable.
Size: This species of coral develops massive and encrusting growth forms. In forereef locations, high-energy sea conditions limit its size to generally less than 20 cm in diameter (Kenyon et al. 2006).
Diet/Ingestion Rates: Corals feed on small plankton and the sugars produced by symbiotic algae.
Predators: Several species of reef fish specialize on coral, including Porites lobata (Cole et al. 2008). Typical corallivorous fishes in Hawaiʻi include the spotted puffer (Arothron meleagris) and the barred filefish (Cantherhines dumerilii) (Jayewardene et al. 2009).
Tissue Data/Bioaccumulation Factors/Toxicity Data:
- Irgarol 1051®, a marine herbicide (anti-fouling compound) was shown to be toxic to coral larvae at concentrations measured in small boat marinas in Oʻahu. Laboratory exposures of 100 ng/L Irgarol caused a reduction in settlement of larvae of Porites hawaiiensis, a Hawaiian coral typical of shady marinas (Knutson et al. 2012).
- Bioaccumulation of trace metals from sediment and seawater were reported in Porites lobata in Malaysia (Mokhtar et al. 2012).
- Coral (Porites astreoides) collected in Puerto Rico were analyzed for PAHs, PCBs, organochlorine pesticides, and metals (Pait et al. 2009). Coral tissues accumulated PAHs, PCBs and trace elements, such as copper and zinc. Generally, corals contained higher levels of alkylated PAHs than the sediments.
- PCBs and metals (arsenic, cadmium, lead, and selenium) were analyzed in coral (Porites lobata) collected from Tern Island in the NWHI (Miao et al. 2000a). Results indicated that coral preferentially accumulated less chlorinated PCBs. Metal concentrations in coral were generally equal to or less than those in sediment. Another coral species (Porites evermanni) collected from Tern Island and Disappearing Island in the French Frigate Shoals was analyzed for PCBs (Miao et al. 2000b).
- Metals (arsenic, cadmium, chromium, copper, lead, and selenium) were analyzed in coral (Porites evermanni) collected from Oʻahu and French Frigate Shoals (Miao et al. 2001). Lead concentrations were greater in samples from French Frigate Shoals than from Oʻahu. Lead may be from activities associated with Navy and USCG occupation of the atoll.
- Scleractinian coral (Stylophora pistillata) obtained from culture tanks in Taiwan were exposed to Aroclor-1254 (Chen et al. 2012) to evaluate short and long term toxicity. No mortality or bleaching was observed during the 96-hour exposure period and delayed effects were not observed in the 50-day recovery period.
- Three species of scleractinian corals were exposed to copper (Bielmyer et al. 2010). Sensitivity to copper varied among the three species.
Conservation Status: Not threatened.
Other notes: Many stony corals are threatened by overgrowth of introduced marine algae (Hawaiʻi Coral Reef Initiative 2002). Other threats include disease, fishing pressures, and nutrient and sediment runoff (Hawaiʻi Coral Reef Initiative 2002). Porites lobata has demonstrated low susceptibility to bleaching (Kenyon et al. 2006).
References
Bielmyer, G.K., Grosell, M., Bhagooli, R., Baker, A.C., Langdon, C., Gillette, P. and T.R. Capo, 2010. Differential effects of copper on three species of scleractinian corals and their algal symbionts (Symbiodinium spp.). Aquatic Toxicology, 97: 125–133.
Chen, T.H., Cheng, Y.M., Cheng, J.O., and Ko, F.C. 2012. Assessing the effects of polychlorinated biphenyls (Aroclor 1254) on a scleractinian coral (Stylophora pistillata) at organism, physiological, and molecular levels. Ecotoxicology and Environmental Safety, 75(1), 207-212.
Cole, A.J., Pratchett, M.S., and G.P. Jones, 2008. Diversity and functional importance of coral-feeding fishes on tropical coral reefs. Fish and Fisheries 9: 286–307.
Friedlander, A., G. Aeby, S. Balwani, B. Bowen, R. Brainard, A. Clark, J. Kenyon, J. Maragos, C. Meyer, P. Vroom and J. Zamzow (2008a). The state of coral reef ecosystems of the Northwestern Hawaiian Islands. In: The State of Coral Reef Ecosystems of the United States and Pacific Freely Associated States: 2008. J.E. Waddell and A.M. Clarke. Silver Spring, MD, NOAA/NCCOS Center for Coastal Monitoring and Assessment’s Biogeography Team: 263-306.
Friedlander, A., G. Aeby, R. Brainard, E. Brown, K. Chaston, A. Clark, P. McGowan, T. Montgomery, W. Walsh, I. Williams and W. Wiltse (2008b). The state of coral reef ecosystems of the main Hawaiian Islands. In: The State of Coral Reef Ecosystems of the United States and Pacific Freely Associated States: 2008. J.E. Waddell and A.M. Clarke. Silver Spring, MD, NOAA/NCCOS Center for Coastal Monitoring and Assessment’s Biogeography Team: 219-261.
Hawaiʻi Coral Reef Initiative. 2002. The First Four Years: Hawaiʻi Coral Reef Initiative Research Program 1998-2002. https://www.hcri.ssri.hawaii.edu/files/education/edu-about-01.pdf
Jayewardene, D., M.J. Donahue, and C. Birkeland. 2009. Effects of frequent fish predation on corals in Hawaiʻi. Coral Reefs 28:499–506
Kenyon, J.C., Vroom, P.S., Page, K.N., Dunlap, M.J., Wilkinson, C. B, and G.S. Aeby. 2006. Community Structure of Hermatypic Corals at French Frigate Shoals, Northwestern Hawaiian Islands: Capacity for Resistance and Resilience to Selective Stressors. Pacific Science 60(2):153-175.
Knutson, S., C.A. Downs and R.H. Richmond. 2012. Concentrations of Irgarol in selected marinas of Oʻahu, Hawaiʻi and effects on settlement of coral larval. Ecotoxicology 21(1): 1-8.
Miao, X.S., Swenson, C., Yanagihara, K., and Q.X. Li, 2000a. Polychlorinated Biphenyls and Metals in Marine Species from French Frigate Shoals, North Pacific Ocean. Arch. Environ. Contam. Toxicol. 38, 464–471.
Miao, X.S., Swenson, C., Woodward, L.A., and Q.X. Li, 2000b. Distribution of polychlorinated biphenyls in marine species from French Frigate Shoals, North Pacific Ocean. The Science of the Total Environment, 257: 17-28.
Miao, X.S., Woodward, L.A., Swenson, C., and Q.X. Li, 2001. Comparative Concentrations of Metals in Marine Species from French Frigate Shoals, North Pacific Ocean. Marine Pollution Bulletin, 42 (11): 1049-1054.
Mokhtar, M.B., Preveena, S.M., Aris, A.Z., Yong, O.C., and A.P. Lim, 2012. Trace metal (Cd, Cu, Fe, Mn, Ni and Zn) accumulation in Scleractinian corals: A record for Sabah, Borneo. Marine Pollution Bulletin, 64: 2556-2563.
Pait, A.S., Jefferey, C.F.G., Caldow, C., Whitall, D.R., Hartwell, S.I., Mason, A.L., and J.D. Christensen, 2009. Chemical contamination in southwest Puerto Rico: A survey of contaminants in the coral Porites astreoides. Caribbean Journal of Science, 45(2-3): 191-203.
Sheppard, A., Fenner, D., Edwards, A., Abrar, M. & Ochavillo, D. 2014. Porites lobata. The IUCN Red List of Threatened Species. Version 2014.2. https://www.iucnredlist.org
Photo Credit: Dr. James P. McVey, NOAA Sea Grant Program and National Oceanic and Atmospheric Administration/Department of Commerce https://www.photolib.noaa.gov/index.html
10 – Black sea cucumber (Holothuria atra)
 Black sea cucumber (Holothuria atra) loli okuhi kuhi |
Native Hawaiian Species: Yes
Habitat: Fourteen species of sea cucumbers are known to occur in Hawaiʻi. They are found in tide pools, on reefs, in bays and lagoons, and in deeper waters (Waikīkī Aquarium 2014d). The black sea cucumber is a common species of tide pools (Waikīkī Aquarium 2014d). The black sea cucumber occurs in sandy habitats in Indonesia and Guam and among coral rubble in Western Australia (Roberts and Bryce 1982).
Locations: The black sea cucumber is the most common holothurian on Hawaiian coral reefs and is found throughout the Indo-Pacific region (Skillings et al. 2014).
Seasonality (year-round resident or migrant): Year-round resident.
Cultural Use (historical and current): None known
Recreational Harvest: Yes, certain species are consumed (Waikīkī Aquarium 2014d).
Commercial Harvest: In the Pacific, H. atra is harvested, although it is a low value species (Skillings et al. 2014). Other species of sea cucumbers such as H. whitmaei are preferred as a food source. There is no regulated sea cucumber fishery in Hawaiʻi (Skillings et al. 2014).
Home Range: The movement of another species of sea cucumber, H. sanctori, found that they traveled between 1.86 and 21.47 meters each day with a mean distance of 11.12 ± 4.24 meters (Navarro et al. 2013). These distances are greater than those observed in other sea cucumber species, such as A. mauritiana (3.02 meters, Graham and Battaglene 2004 [as cited in Navarro et al. 2013]) and P. californicus (3.93 meters, Da Silva et al. 1986 [as cited in Navarro et al. 2013]). In areas with numerous potential refuges, the sea cucumbers do not return to the same shelters (Navarro et al. 2013). Sea cucumbers may also move accidents as they can become dislodged from the ocean floor by waves (Roberts and Bryce 1982).
Size/Body Weight: The black sea cucumber may reach up to 12 inches in length (Waikīkī Aquarium 2014e).
Diet/Ingestion Rates: Sea cucumbers feed on organic matter in water and sediment (Waikīkī Aquarium 2014e).
Predators: Humans and marine invertebrates such as crabs and gastropods (Kropp 1982). The black sea cucumber releases a red substance to defense itself against predators (Waikīkī Aquarium 2014e); the substance is toxic to marine fish but is not effective against crustaceans (Yamanouchi 1955, Bakus 1968 [as cited in Kropp 1982]).
Tissue Data (or other use in ecological or human health risk assessment)/Bioaccumulation Factors: None identified.
Conservation Status: Not threatened.
References
Kropp, R.K., 1982. Responses of Five Holothurian Species to Attacks by a Predatory Gastropod, Tonna perdu. Pacific Science, 36 (4): 445-452.
Navarro, P.G., García-Sanz, S., Barrio, J.M., and F. Tuya, 2013. Feeding and movement patterns of the sea cucumber Holothuria Sanctori. Mar Biol, 160: 2957–2966.
Roberts, D. and C. Bryce, 1982. Further Observations on Tentacular Feeding Mechanisms in Holothurains.J. Exp. Mar. Biol. Ecol., 59: 151-163.
Skillings, D.J., Bird, C.E., and R.J. Toonen, 2014. Comparative population structure of two edible Indo-Pacific coral reef sea cucumbers (Echinodermata: Holothuroidea). Bull Mar Sci. 90(1):359–378.
Waikīkī Aquarium, 2014d. Sea cucumbers. Animal Guide. https://www.waikikiaquarium.org/experience/animal-guide/invertebrates/echinoderms/sea-cucumbers/ Accessed September 2, 2014.
Waikīkī Aquarium, 2014e. Black sea cucumber. Animal Guide. https://www.waikikiaquarium.org/experience/animal-guide/invertebrates/echinoderms/sea-cucumbers/black-sea-cucumber/ Accessed September 3, 2014.
Photo Credit: “Holothura atra. loli okuhi kuhi. black sea cucumber” Photo taken by Scott Godwin, National Park Service. https://www.botany.hawaii.edu/basch/uhnpscesu/htms/kahoinvr/fish_pops/holothur/wana02.htm
11 – Goatfish (Mulloides)
 Yellowfin Goatfish (Mulloides vanicolensis) Weleʻula |
Native Hawaiian Species: Yes
Taxonomy: Family Mullidae
Habitat: Goatfish comprise a major component of Hawaiian reefs. They are named for the chemosensory barbels on their chins that they use to detect benthic invertebrate prey (Szabó et al. 2014). All goatfish are expected to be exposed to sediment. The yellowtail goatfish is most abundant near primary reefs. This species tends to remain low in the water column; most individuals observed off Oʻahu were less than 2 meters above the substrate. Other species of goatfish occur in slightly different habitats: the manybar goatfish ventures farther from the primary reef, but also stays low in the water column. The white goatfish lives in large schools higher in the water column (Schumacher and Parrish, 2004). The white-saddle goatfish and white goatfish forage at night on sandflats (Meyer et al. 2000, Holland et al. 1993).
Locations: Goatfish occur throughout the Hawaiian Islands and Indo-Pacific region.
Seasonality: Year-round resident.
Cultural Use (historical and current): Some species of goatfish, such as the kūmū, were historically used in religious ceremonies as offerings (Titcomb 1972 [as cited in Meyer et al. 2000], Waikīkī Aquarium 2014).
Recreational Harvest: Yes. Goatfishes may be harvested by spear fishing (Meyer 2010). They are a popular food in Hawaiʻi (Waikīkī Aquarium 2014). Recreational catch of wekeʻā (yellowstripe goatfish) constitutes the second largest landings within the state, with over 1.0 million kg in reported landings between 2005 and 2009, and 1.2 million kg reported for all species of Mullidae during that period (WPRFMC 2011). The arrival of large numbers of juvenile goatfish, oʻama, is a popular event for recreational fishermen, who can be seen all around the islands in late summer standing in the shallow waters, catching the small fish for live bait or to eat.
Commercial Harvest: The mean archipelago-wide commercial catch of goatfish between 2005 and 2009 was 5,395 kg, which is down 80 percent from the long-term (1966-2009) average (WPRFMC 2011). (Hawaiʻi DLNR 2011) reported on commercial landings of almost 50,000 pounds of goatfishes (all species combined), including 9400 pounds of kumu and 5400 pounds of moano.
Home Range: Movement patterns indicate that goatfishes are mobile and have a less well-defined home range compared to surgeonfish and parrotfish (Meyer et al. 2010). In an investigation of several reef fishes (parrotfishes, surgeonfishes, and goatfishes) most fish ranged along 0.2 to 1.6 km of coastline (Meyer et al. 2010). The white-saddle goatfish range across 9,070 to 35,163 square meters in 3 to 14 days (Meyer et al. 2000). The white goatfish ranged across 8,267 square meters at night and 2,533 square meters during the day (Holland et al. 1993).
Size/Body Weight: Adult goatfishes range from 23 to 40 cm in length (Waikīkī Aquarium, 2014).
Diet/Ingestion Rates: Goatfishes feed on benthic organisms in sand and mud around reefs. Their diet includes bottom-dwelling invertebrates such as worms, crustaceans, small mollusks, brittle stars and heart urchins (Waikīkī Aquarium, 2014). Some goatfish species will also feed on small fishes (Waikīkī Aquarium, 2014).
Predators: Humans, marine mammals, other goatfishes, and the greater amberjack (Seriola dumerili) (Schumacher and Parrish 2004).
Available Tissue Data/Bioaccumulation Factors:
- Constituents in 21 samples of goatfish fillets from reference locations on the Waiʻanae Coast of Oʻahu (fall and spring samples) (U.S. Army Corps of Engineers [USACE] 2012):
- Inorganic Constituents: Antimony, Arsenic, Barium, Cadmium, Chromium, Cobalt, Copper, Lead, Mercury, Nickel, Selenium, Strontium, Thallium, Uranium, Vanadium, and Zinc
- Organic Constituents: PCBs, several energetic compounds.
- Composite samples of manybar goatfish (Parupeneus multifasciatus) were collected from nearshore waters at Mākua (2 samples) and the background location Sandy beach (1 sample) (see Figure 2-1 and Table 2-2 in Tetra Tech 2009). Samples were analyzed for dioxins/furans, VOCs SVOCs, organochlorine pesticides, explosives, and metals (Table 3-1 in Tetra Tech 2009). The samples had 3.9 to 9.6 mg/kg total lipids and 65.8 to 70 percent moisture. Detected constituents included the following:
- Inorganic Constituents: Aluminum, Arsenic, Barium, Cadmium, Chromium, Cobalt, Copper, Iron, Lead, Manganese, Mercury, Methyl Mercury, Selenium, Silver, Vanadium, and Zinc
- Organic Constituents: Dioxins/furans, Acetone, m+p-Xylenes, bis(2-ethylhexyl)phthalate, di-n-Butylphthalate, Aldrin, alpha-BHC, 4,4′-DDT, Heptachlor, Heptachlor epoxide, Nitroglycerin, Perchlorate, and RDX
- 7 whole-body samples of the bandtail goatfish (Upeneus taeniopterus) were collected from Pearl Harbor and analyzed in 1996 for metals, butyltins, polycyclic aromatic hydrocarbons, semi-volatile organic compounds, organochlorine pesticides, PCBs, dioxins/furans, herbicides, triazine pesticides, 2,4,6-trinitrotoluene , and other ordnance chemicals (see Subsection 4.3 and Table 4-3 in Navy 2007b.) These data along with data from tilapia were used to estimate bioaccumulation in bottom fish (see Subsection 6.1.3 in Navy 2007).
- 60 whole fish samples (composites or single specimens) of the bandtail goatfish (Upeneus taeniopterus) were collected from Pearl Harbor in 2009 and analyzed for metals, PCBs, pesticides, and dioxins/furans. Fish were 6.5 to 12.5 inches total length. Concentrations of these constituents in goatfish were reported to have decreased since previous sampling in 1996 (see Subsection 3.2.3 in Navy 2010).
- Whole body samples and whole body composite samples were collected from the Kure Lagoon (length 7 to 12 inches and weight 5.75 to 14.5 grams) in 2008 and analyzed for PCBs, metals (arsenic, cadmium, chromium, lead, and mercury), and percent lipids (see Figure 3-3 and Tables 3-5 and B-10 to B-12 in Element Environmental 2009). Lead and PCBs analyzed for in whole body goatfish samples from Tern Island and Midway Atoll, which were used as reference locations (Element Environmental 2009).
- Metal concentrations in goatfish from Honolua Bay compared to other geographical areas (see Table 2 and 4 in Hedouin et al. 2011).
- Yellowfin goatfish (Mulloidichthys vanicolensis) collected from Disappearing Island in French Frigate Shoals were analyzed for PCBs and metals (arsenic, cadmium, chromium, copper, lead, selenium, and zinc) (Miao et al. 2000; Miao et al. 2001).
Conservation Status: Not threatened.
References Cited:
Element Environmental. 2009. Site Investigation Report: Evaluation of Green Island Landfill and Reburial Pit, Former U.S. Coast Guard LORAN Station Kure, Northwest Hawaiian Islands
Hawaiʻi Department of Land and Natural Resources. 2011. Commercial Marine Landings Summary Trend Report: Calendar Year 2011. 19 pages. https://dlnr.hawaii.gov/dar/files/2014/04/cmlstr2011.pdf
Hédouin, L., Metian, M., and R.D. Gates, 2011. Ecotoxicological approach for assessing the contamination of a Hawaiian coral reef ecosystem (Honolua Bay, Maui) by metals and a metalloid. Marine Environmental Research, 71: 149-161.
Holland, K.N., Peterson, J.D., Lowe, C.G., and B.M. Wetherbee, 1993. Movements, Distribution and Growth Rates of the White Goatfish Mulloides flavolineatus in a Fisheries Conservation Zone. Bulletin of Marine Science. 52(3): 982-992.
Meyer C.G., Holland, K.N., Wetherbee, B.M. and C.G. Lowe, 2000. Movement patterns, habitat utilization, home range size and site fidelity of whitesaddle goatfish, Parupeneus porphyreus, in a marine reserve. Environmental Biology of Fishes 59: 235–242.
Meyer, C.G., Papastamatiou, Y.P., and T.B. Clark, 2010. Differential movement patterns and site fidelity among trophic groups of reef fishes in a Hawaiian marine protected area. Marine Biology 157:1499–1511.
Miao, X.S., Swenson, C., Woodward, L.A., and Q.X. Li, 2000. Distribution of polychlorinated biphenyls in marine species from French Frigate Shoals, North Pacific Ocean. The Science of the Total Environment, 257: 17-28.
Miao, X.S., Woodward, L.A., Swenson, C., and Q.X. Li, 2001. Comparative Concentrations of Metals in Marine Species from French Frigate Shoals, North Pacific Ocean. Marine Pollution Bulletin, 42 (11): 1049-1054.
Navy. 2007. Step 7 Baseline ERA, Pearl Harbor: Sediment Remedial Investigation.
Navy (Department of the Navy), Naval Facilities Engineering Command Pacific. 2010. Draft Final: Remedial Investigation Addendum, Pearl Harbor Sediment. Pearl Harbor, Hawaiʻi. 604 pages.
Schumacher, B.D. and J.D. Parrish, 2004. Spatial relationships between an introduced snapper and native goatfishes on Hawaiian reefs. Biological Invasions. 7: 925–933.
Szabé, Z., B. Snelgrove, M.T. Craig, L.A. Rocha and B.W. Bowen (2014). “Phylogeography of the manybar goatfish, Parupeneus multifasciatus, reveals isolation of the Hawaiian Archipelago and a cryptic species in the Marquesas Islands.” Bulletin of Marine Science 90(1): 493-512.
Tetra Tech, Inc. 2009. Marine Resources Study Field Sampling Results and Risk Assessment, Mākua Military Reservation, Oʻahu, Hawaiʻi.
U.S. Army Corps of Engineers. 2012. Ecological Risk Assessment and HHRA (Appendices to RI): Ordnance Reef (Site HI-06) Waiʻanae, Oʻahu, Hawaiʻi.
Waikīkī Aquarium, 2014. Goatfishes. Animal Guide. https://www.waikikiaquarium.org/experience/animal-guide/fishes/goatfishes/goatfishes/ Accessed September 2, 2014.
Western Pacific Regional Fishery Management Council (WPRFMC). 2011. Hawaiʻi Archipelago Fishery Ecosystem Plan 2009 Annual Report. January 2011. 49 pp. https://www.wpcouncil.org/documents/Reports/annualreports/FINAL%20Hawaiʻi%20Archipelago%20FEP%202009%20Annual%20Report.pdf Updated URL: https://www.wpcouncil.org/wp-content/uploads/2019/08/FINAL-Hawaii-Archipelago-FEP-2009-Annual-Report.pdf
Other Recommended References
Beets, J., E. Brown, and A. Friedlander. 2010. Inventory of marine vertebrate species and fish-habitat utilization patterns in coastal waters off four national parks in Hawaiʻi. Pacific Cooperative Studies Unit Technical Report 168. University of Hawaiʻi at Mānoa. Department of Botany. Honolulu, HI. 55 pg. https://manoa.hawaii.edu/hpicesu/techr/168/v168.pdf Updated URL: https://scholarspace.manoa.hawaii.edu/handle/10125/15274
Parrish, J., G. Smith, and J. Norris. 1990. Resources of the marine waters of Kaloko-Honokōhau National Historical Park. Cooperative National Park Resources Study Unit, Technical Report 74. Department of Botany, University of Hawaiʻi. Honolulu, HI. 118 pp. https://manoa.hawaii.edu/hpicesu/techr/074.pdf Updated URL: https://scholarspace.manoa.hawaii.edu/handle/10125/5886
Names of fishes in this species profile are taken from Fishes of Kaloko-Honokōhau National Historical Park (https://www.botany.hawaii.edu/basch/uhnpscesu/htms/kahofish/NSAlista_g.htm), which cites Reef and Shore Fishes of the Hawaiian Islands (Randall 2007).
Photo Credit: Bryan Harry (yellowfin and manybar goatfishes); Peg Bethany (yellowstripe goatfish) https://www.botany.hawaii.edu/basch/uhnpscesu/htms/kahofish/plates/pictz05.htm Updated URL: https://www.botany.hawaii.edu/basch/uhnpscesu/htms/kahofish/fish_pops/mullid/goatfish02.htm
12 – Hawaiian flagtail (Kuhlia sandvicensis)
 Hawaiian flagtail (Kuhlia sandvicensis) Aholehole |
Native Hawaiian Species: yes (McRae et al. 2011)
Habitat: Marine and freshwater habitats (Benson and Fitzsimons 2002). Two recognized forms of Kuhlia occur in Hawaiʻi (K. sandvicensis and K. xenura); both live in schools on or near coral reefs as adults, and spawn in marine or estuarine waters (see other notes below). K. sandvicensis occurs predominately in marine habitats, but juvenile K. xenura are known from freshwater streams, estuaries, on reef flats, along rocky shorelines, and in tide-pool habitats. Along rocky shorelines and in tidepools, K. sandvicensis uses microhabitats characteristic of high-energy surge zones-deep areas close to the open ocean that have high salinities. K. xenura occurs along shallower rocky shorelines, typically in lower salinities; it may range farther inland, including protected tide pools with low salinities (McRae et al. 2011).
Locations: All islands (Hawaiʻi DLNR 2005)
Cultural Use (historical and current): These species were culturally important to the ancient Hawaiian people and were often used in religious ceremonies (Titcomb 1972 [as cited in McRae 2011]).
Recreational Harvest: Important food fish in the Hawaiian Islands (Gosline and Brock 1965 [as cited in McRae, 2011]).
Commercial Harvest: Commercial landings for both Kuhlia species in the Main Hawaiian Islands averaged about 1,350 kilograms (3,000 pounds) a year in recent years; landings dropped to 900 kilograms (2,000 pounds) in 2003, the most recent year for which data are available (Hawaiʻi DLNR 2005).
Home Range: No data found.
Size/Body Weight: The aholehole can grow to about 30 cm total length (Gosline and Brock 1965, as cited in McRae, 2011). No body weight data were found.
Diet/Ingestion Rates: The aholehole consumes a variety of small items, consisting of algae, invertebrates, and, insects (Tester and Trefz 1954). No food or sediment ingestion rates were found.
Predators: Humans. No other specific predators were reported.
Tissue Data: One composite sample of Hawaiian flagtail (Kuhlia sandvicensis) collected from the Mākua north muliwai was analyzed for dioxins/furans, VOCs, SVOCs, organochlorine pesticides, explosives, and metals (see Figure 2-1 and Tables 2-2 and 3-1 in Tetra Tech 2009). The sample had 6.4 percent total lipids and 72.3 percent moisture. Detected constituents included the following:
- Inorganic Constituents: Aluminum, Antimony, Arsenic, Barium, Cadmium, Chromium, Cobalt, Copper, Iron, Lead, Manganese, Mercury, Methyl Mercury, Selenium, Silver, Vanadium, and Zinc
- Organic Constituents: Dioxins/furans, di-n-Butylphthalate, Aldrin, 4,4′-DDT, Heptachlor epoxide
Conservation Status: None, but regulations set minimum catch size at five inches (Hawaiʻi DLNR 2005).
Other Notes:
Two morphotypes (based primarily on eye size) had long been noted by local fishermen and biologists, before 2001, but only one species, K. sandvicensis (Steindachner 1876) was recognized in the scientific literature until recently (McRae, 2011). Randall and Randall (2001 [as cited in McRae 2011]) published a revision of the genus that, effectively “split” K. sandvicensis into two species. The “big-eyed” (colloquial) morphotype was assigned the name K. xenura (Jordan & Gilbert 1882); this species is believed to be endemic to the Hawaiian Islands. Meanwhile, the “small-eyed” morphotype, even though less frequently observed in what were formerly known as the Sandwich Islands, retained the name K. sandvicensis.
References
Benson, L.K. & Fitzsimons, J.M. 2002. Life history of the Hawaiian fish Kuhlia sandvicensis as inferred from daily growth rings of otoliths. Environmental Biology of Fishes, 65, 131–137.
Hawaiʻi Department of Land and Natural Resources (DLNR), 2005. Marine Fishes Hawaiian flagtail Āholehole Kuhlia xenura, Hawaiʻi’s Comprehensive Wildlife Conservation Strategy. https://dlnr.hawaii.gov/wildlife/files/2013/09/Fact-Sheet-aholehole.pdf
McRae, M.G., McRae, L.B., & Fitzsimons, J.M. 2011. Habitats used by juvenile flagtails (Kuhlia spp.; Perciformes: Kuhliidae) on the Island of Hawaiʻi. Pacific Science, 65 (4), 441 – 450
Tester, A.L., & Trefz, S.M. 1954. The food of the Aholehole, Kuhlia sandvicensis (Steindachner) in Hawaiian waters. Pacific Science, 8, 3 – 10.
Tetra Tech, Inc. 2009. Marine Resources Study Field Sampling Results and Risk Assessment, Mākua Military Reservation, Oʻahu, Hawaiʻi
Randall, J.E., and H.A. Randall. 2001. Review of the fishes of the genus Kuhlia (Perciformes: Kuhliidae) of the central Pacific. Pac. Sci. 55:227 – 256, as cited in McRae, 2011.
Photo Credit: “Kuhlia sandvicensis” by National Park Service photo – Bryan Harry – https://www.nps.gov/archive/kaho/KAHOckLs/KAHOreef/flagtail2.htm. Updated URL: https://www.botany.hawaii.edu/basch/uhnpscesu/htms/KAHOckLs/KAHOreef/flagtail2.htm Licensed under Public domain via Wikimedia Commons – https://commons.wikimedia.org/wiki/File:Kuhlia_sandvicensis.jpg#mediaviewer
13 – Convict tang (Acanthurus triostegus)
 Convict tang (Acanthurus triostegus) Manini |
Native Hawaiian Species: Yes
Habitat: The convict tang is common on coral reefs, but also occurs in tide pools and other nearshore habitats. This species occurs across the Indo-Pacific in temperatures from 24 to 26 ºC and depths of 0 to 45 m (Waikīkī Aquarium 2014; Gamoke 2012 and references within).
Locations: The convict tang occurs on all Hawaiian islands. It was reported among the top 10 most common species within most of the Marine Life Conservation Districts (MLCD) in the state (Friedlander et al. 2006).
Cultural Use (historical and current): Used as food by early Hawaiians; various life stages of this fish are known by separate Hawaiian names (Waikīkī Aquarium 2014).
Recreational Harvest: Convict tangs are popular recreational fish in Hawaiʻi (McIlwain 2012, Longenecker et al. 2008). An average of 84,000 pounds were harvested annually between 2004 and 2011 throughout the state; in some years, the recreational catch occasionally exceeds 100,000 pounds (Williams and Ma 2013).
Commercial Harvest: Hawaiʻi DLNR reported that about 18,000 pounds of manini were landed by commercial fisheries in 2011, the last year for which records are available (DLNR 2011).
Home Range: No published information on the home range or territory size of the convict tang is available (Gamoke, 2012). An early tagging study indicated that juveniles remain in the same tidepool to which they originally recruit, then move onto a nearby reef to live out their adult lives. Migration was not observed, although some logistical problems with the tagging study limited the interpretation of the data (Randall 1961). However, data for other surgeonfish with similar life histories indicate that individuals generally move between foraging locations and spawning locations every two or three days. Some convict tangs were reported to migrate up to 2 km to reach spawning sites on the seaward side of reefs in Hawaiʻi (Domeier and Colin, 1997, as cited in Gamoke, 2012)
Size/Body Weight: The convict tang averages about 17 cm total length; a typical adult has a total length of 20 cm and weighs 200 grams (Longenecker et al. 2008). Life expectancy is estimated to be at least 4 years (Longenecker et al. 2008).
Diet/Ingestion Rates: The adult convict tang is herbivorous, grazing on fine, filamentous algae growing on rocks and corals. It will not take animal food even when deprived of other food under laboratory conditions (Randall 1961). It is often observed feeding on algae-covered rocks where freshwater enters nearshore waters (McIlwain 2012). Sediment ingestion by the manini is virtually zero. Unlike other surgeonfishes that ingest coarse sediment to aid digestion in their thick-walled stomachs, the thin-walled stomachs of manini were completely devoid of sediment (Randall 1961).
Predators: Juvenile manini are consumed by many larger fishes, but adults are thought to be relatively free from predation except by humans (Randall 1961). Eagle rays are also known to feed on manini gametes, which are released unguarded into the water column (Gamoke 2012 and references within).
Tissue Data (or other use in ecological or human health risk assessment)/Bioaccumulation factors:
- Whole body samples were collected at Kure Atoll at the site of a former Coast Guard LORAN Station (Length 5 to 8 inches and Weight 3.25 to 7 grams) in 2008 and were analyzed for PCBs, metals (arsenic, cadmium, chromium, lead, and mercury), and percent lipids (see Figure 3-3 and Tables 3-5 and B-10 to B-12 in Element Environmental 2009). Reference tissue concentrations in manini were reported from other NWHI locations.
- Whole body samples were collected from locations within Cocos Lagoon, Guam (sample coordinates provided in Table 3-4 in Element Environmental 2010). This fish is typically eaten whole. Samples (45 to 96 grams) were analyzed for PCBs with a mean concentration of 13.74 µg/kg (see Table 6-1 in Element Environmental 2010).
- Convict tang (Acanthurus triostegus) were collected from the near shore area at ʻĪlio Point, Molokaʻi in 2010 from locations near a debris pile site (see Figure 9 in ESI 2012). Species weighted 25 to 173 grams and were 10.4 to 19.2 cm in length (see Table 2-12 in ESI 2012). Samples were analyzed for metals and PCBs (see Tables 2-18 and 2-19 in ESI 2012).
- Convict tang (Acanthurus triostegus) collected from Disappearing Island in French Frigate Shoals were analyzed for PCBs and metals (arsenic, cadmium, chromium, copper, lead, selenium, and zinc) (Miao et al. 2000; Miao et al. 2001).
Conservation Status: No special status (Gamoke 2012; McIlwain 2012).
Other Notes: The convict tang is considered by some to be a subspecies endemic to Hawaiʻi, Acanthurus triostegus sandvicensis (Longenecker et al. 2008; Randall 1961).
References:
Element Environmental. 2009. Site Investigation Report: Evaluation of Green Island Landfill and Reburial Pit, Former U.S. Coast Guard LORAN Station Kure, Northwest Hawaiian Islands.
Element Environmental. 2010. 2010 Follow-On Environmental Investigation Former LORAN Station Cocos Island, Cocos Island, Guam.
ESI (Environmental Science International), 2012. Removal Action Report. Former U.S. Coast Guard Long Range Navigation (LORAN) Station, ʻĪlio Point, Molokaʻi, Hawaiʻi. July.
Friedlander, A.M., E. Brown, M.E. Monaco and A. Clark. 2006. Fish Habitat Utilization Patterns and Evaluation of the Efficacy of Marine Protected Areas in Hawaiʻi: Integration of NOAA Digital Benthic Habitats Mapping and Coral Reef Ecological Studies. Silver Spring, MD: 213 pages.
Gamoke, R. 2012. Acanthurus triostegus, (On-line), Animal Diversity Web. Accessed August 21, 2014 at https://animaldiversity.ummz.umich.edu/accounts/Acanthurus_triostegus/
Hawaiʻi Department of Land and Natural Resources (DLNR) 2011. Commercial Marine Landings Summary Trend Report: Calendar Year 2011. 19 pages. https://dlnr.hawaii.gov/dar/files/2014/04/cmlstr2011.pdf
Longenecker, K., R. Langston, and R. Eble. 2008. Growth, mortality, and reproduction of Manini, Acanthurus triostegus sandvicensis. Contribution 2006-008 to the Hawaiʻi biological Survey. 23 pages. https://hbs.bishopmuseum.org/publications/pdf/manini.pdf
McIlwain, J., Choat, J.H., Abesamis, R., Clements, K.D., Myers, R., Nanola, C., Rocha, L.A., Russell, B. & Stockwell, B. 2012. Acanthurus triostegus. The IUCN Red List of Threatened Species. Version 2014.2. https://www.iucnredlist.org. Downloaded on 22 August 2014.
Miao, X.S., Swenson, C., Woodward, L.A., and Q.X. Li, 2000. Distribution of polychlorinated biphenyls in marine species from French Frigate Shoals, North Pacific Ocean. The Science of the Total Environment, 257: 17-28.
Miao, X.S., Woodward, L.A., Swenson, C., and Q.X. Li, 2001. Comparative Concentrations of Metals in Marine Species from French Frigate Shoals, North Pacific Ocean. Marine Pollution Bulletin, 42 (11): 1049-1054.
Randall JE. 1961. A contribution to the biology of the convict surgeonfish of the Hawaiian Islands, Acanthurus triostegus sandvicensis. Pac Sci 15(2): 215-272. https://scholarspace.manoa.hawaii.edu/handle/10125/7992
Waikīkī Aquarium. 2014. Convict Tang. https://www.waikikiaquarium.org/experience/animal-guide/fishes/surgeonfishes/convict-tang/
Williams, I. and I. Ma. 2013. Estimating Catch Weight of Reef Fish Species Using Estimation and Intercept Data from the Hawaiʻi Marine Recreational Fishing Survey. National Marine Fisheries Service, Pacific Islands Fisheries Science Center Administrative Report H-13-04. 53 pages. November.
Photo Credit: “Convict Surgeonfish, Acanthurus triostegus” by brian.gratwicke – Convict Surgeonfish, Acanthurus triostegus. Licensed under Creative Commons Attribution 2.0 via Wikimedia Commons – https://commons.wikimedia.org/wiki/File:Convict_Surgeonfish,_Acanthurus_triostegus.jpg#mediaviewer
14 – Pacific Sergeant (Abudefduf abdominalis)
 Pacific Sergeant (Abudefduf abdominalis) Mamo |
Native Hawaiian Species: Yes, endemic to Hawaiʻi (Waikīkī Aquarium 2014).
Habitat: Common marine reef fish; non-migratory; depth range 1 to 50 m (Lieske and Myers 1994 [as cited in Froese and Pauly 2011]). Large aggregations occur in quiet waters over rocky bottoms on inshore and offshore reefs; juveniles frequent surge and tide pools (Waikīkī Aquarium 2014, Breder and Rosen 1966). Aggregations of Hawaiian sergeants swarm high off the bottom during the day to feed on plankton, then settle to the bottom during the night. Spawning occurs almost year round, but is most common from January to June (Hoover 2003).
Locations: Endemic to Hawaiʻi; widespread within MHI and NWHI (Waikīkī Aquarium 2014).
Cultural Use (historical and current): None known.
Recreational Harvest: Taken as food by Hawaiians (Titcomb 1972 [as cited in Froese and Pauly 2011]).
Commercial Harvest: None
Home Range: Aggregations of adults generally feed in water column above spawning substrate. Males are territorial around nest sites. Juveniles occur in tide pools prior to joining adult population (Hoover 2003, Waikīkī Aquarium 2014).
Size/Body Weight: 30.0 cm total length (Lieske and Myers 1994 [as cited in Froese and Pauly 2011]). Up to 25 cm, but usually smaller (Hoover 2003, Waikīkī Aquarium 2014).
Diet/Ingestion Rates: Feed in the water column on a variety of algae and zooplankton (Froese and Pauly 2011, Hoover 2003). No food or sediment ingestion rates were found.
Predators: Hawaiian sergeant eggs provide a significant food source for other fish (including milletseed butterflyfish, raccoon butterflyfish, and black triggerfish) (Hoover 2003).
Tissue Data (or other use in ecological or human health risk assessment)/Bioaccumulation factors:
- Composite samples of blackspot sergeant (Abudefduf sordidus) were collected from the nearshore waters at Mākua (1 sample) and the background location at Sandy Beach (1 sample) (see Figure 2-1 and Table 2-2 in Tetra Tech 2009). Samples were analyzed for dioxins/furans, VOCs, SVOCs, organochlorine pesticides, explosives, and metals (see Table 3-1 in Tetra Tech 2009). The samples had 2.6 to 9.09 percent lipids and 69.3 to 71.2 percent moisture. Detected constituents included the following:
- Inorganic Constituents: Aluminum, Antimony, Arsenic, Barium, Cadmium, Chromium, Cobalt, Copper, Iron, Lead, Manganese, Mercury, Methyl Mercury, Selenium, Silver, Thallium, Vanadium, and Zinc
- Organic Constituents: Acetone, m+p-Xylenes, di-n-Butylphthalate, delta-BHC, 4,4′-DDT, Heptachlor epoxide, and Perchlorate
- Whole body samples of sergeant major (Abudufduf abdominalis) were collected from locations within Cocos Lagoon, Guam (sample coordinates provided in Table 3-4 in Element Environmental 2010). This fish is typically eaten whole. Samples (60 to 120 grams) were analyzed for PCBs with a mean concentration of 123.76 µg/kg (see Table 6-1 in Element Environmental 2010).
Conservation Status: No special status
Other Notes: Abudefduf vaigiensis, the Indo-Pacific damselfish, has appeared in Hawaiian waters in the past two decades, and has been shown to hybridize with the Hawaiian sergeant (Maruka and Peyton 2007, Maruka et al. 2007). Based on life history characteristics and field observations, hybridization is expected to continue and to possibly lead to replacement of the endemic Hawaiian sergeant over time (Maruka and Peyton 2007, Maruka et al. 2007).
References
Breder, C.M. and D.E. Rosen. 1966. Modes of Reproduction in Fishes. Garden City, Ney York. , Natural History Press. 941 pages.
Element Environmental. 2010. 2010 Follow-On Environmental Investigation Former LORAN Station Cocos Island, Cocos Island, Guam.
Froese, R. and D. Pauly. Editors. 2011. FishBase. World Wide Web electronic publication. https://www.fishbase.org
Hoover, J.P. 2003. Hawaiʻisfishes.com website: Fish Of The Month – June 2003, Hawaiian Sergeant Abudefduf abdominalis. https://www.Hawaiisfishes.com/fish_of_month/past_fom/fom_06_03.htm
Maruska, K.P. and K.A. Peyton. 2007. “Interspecific spawning between a recent immigrant and an endemic damselfish (Pisces : Pomacentridae) in the Hawaiian Islands.” Pacific Science 61 (2): 211-221.
Maruska, K.P., K.S. Boyle, L.R. Dewan and T.C. Tricas (2007). “Sound production and spectral hearing sensitivity in the Hawaiian sergeant damselfish, Abudefduf abdominalis.” Journal of Experimental Biology 210(22): 3990-4004.
Tetra Tech, Inc. 2009. Marine Resources Study Field Sampling Results and Risk Assessment, Mākua Military Reservation, Oʻahu, Hawaiʻi.
Waikīkī Aquarium. 2014. Hawaiian sergeant. https://www.waikikiaquarium.org/experience/animal-guide/fishes/damselfishes/Hawaiian-sergeant/
Photo Credit: National Park Service, Brian Harry https://www.botany.hawaii.edu/basch/uhnpscesu/htms/kahofish/fish_pops/pomacenth/damsel01.htm
15 – Mozambique tilapia (Oreochromis mossambicus)
 Mozambique tilapia (Oreochromis mossambicus) |
Native Hawaiian Species: No. Oreochromis mossambicus was introduced in 1951 around Oʻahu (Coles et al. 1999, Randall 1987). The blackchin tilapia (Sarotherodon melanotheron) has also become established in Hawaiian waters (Randall 1987).
Habitat: These tilapia occur in diverse habitats throughout Hawaiʻi including harbors, streams, estuaries, low wetlands, and reservoirs or ponds of Hawaiʻi (Coles 1999, USGS 2013). Tilapia can tolerate a range of salinities, temperatures, and dissolved oxygen levels (MacKenzie and Bruland 2012). O. mossambicus occurs in high salinities of atoll lagoons, where it nests in the calm sandy areas (Jubb 1967 [as cited in Russell et al. 2012], Lobel 1980 [as cited in ISSG 2014]).
Locations: Established throughout the Hawaiian Islands (Randall 1987).
Seasonality (year-round resident or migrant): Year-round resident.
Cultural Use (historical and current): None identified.
Recreational Harvest: Limited.
Commercial Harvest: Yes. Tilapia were introduced as potential aquaculture species (Yamamoto and Tagawa 2000 [as cited in MacKenzie and Bruland 2012]). About 3,000 pounds of tilapia (undefined species) were landed by commercial fisheries in 2011 (Hawaiʻi DLNR 2011).
Home Range: Not identified.
Size/Body Weight: Approximately 40 cm total length (Skelton 1993 [as cited in USGS 2013]). Adult tilapia collected from a stream and canal in Oʻahu weighed approximately 200 grams (Yang et al. 2008).
Diet/Ingestion Rates: Tilapia are opportunistic omnivores, consuming vegetation, algae, plankton, invertebrates, and fish (Arthington and Bluhdorn 1994, Bruton and Boltt 1975, De Moor et al. 1986, De Silva et al. 1984, Fuselier 2001, Jameson 1991, Komarkova and Tavera 2003, Mathavan et al. 1976, Wager and Rowe-Rowe 1972 [as cited in Russell et al. 2012]). A laboratory investigation estimated a maximum ingestion rate of 3.89 x 10⁹ µm³ g⁻¹ h⁻¹ for another tilapia species consuming blue-green algae (Northcott et al. 1991).
Predators: Humans and predatory fishes such as barracuda (Randall 1987).
Tissue Data (or other use in ecological or human health risk assessment)/Bioaccumulation Factors:
- PCBs in tilapia in streams on Oʻahu were in the middle range of levels found worldwide in freshwater and marine waters (Yang 2008).
- 8 whole-body samples of Oreochromis mossambicus were collected from Pearl Harbor and analyzed in 1996 for metals, butyltins, polycyclic aromatic hydrocarbons, semi-volatile organic compounds, organochlorine pesticides, PCBs, dioxins/furans, herbicides, triazine pesticides, 2,4,6-trinitrotoluene, and other ordnance chemicals (see Subsection 4.3 and Table 4-3 in Navy 2007b). These data along with data from goatfish were used to estimate bioaccumulation of by bottom fish (see Subsection 6.1.3 in Navy 2007b).
- Composite samples of tilapia (Talapia zillii, T. rendalii, Oreochromis macrochir, O. mossambicus, Sarotherdon melanotheron, Melanotheron) were collected from Mākua North Muliwai (3 samples), Mākua South Muliwai (3 samples) and the background location Nanakuli Muliwai (3 samples) (see Figure 2-1 and Table 2-2 in Tetra Tech 2009). Samples were analyzed for dioxins/furans, VOCs, SVOCs, organochlorine pesticides, explosives, and metals (see Table 3-1 in Tetra Tech 2009). Lipid content of tilapia samples ranged from 3.3 to 5.1 mg/kg total lipids and 13.9 to 21.3 percent lipid. Total moisture in tilapia samples ranged from 71.3 to 74.3 percent. Detected constituents included the following:
- Inorganic Constituents: Aluminum, Antimony, Arsenic, Barium, Cadmium, Chromium, Cobalt, Copper, Iron, Lead, Manganese, Mercury, Methyl Mercury, Selenium, Silver, Thallium, Vanadium, and Zinc
- Organic Constituents: Dioxins/furans, Acetone, m+p-Xylenes, Bis(2-ethylhexyl)phthalate, di-n-Butylphthalate, beta-BHC, delta-BHC, gamma-BHC, 4,4′-DDT, Heptachlor epoxide, and Perchlorate
Conservation Status: Invasive nonindigenous species.
Other Notes: Tilapia are considered threats to native species such as the mullet (Mugil cephalus) based on competition for food (Randall 1987).
References
Coles, S.L., DeFelice, R.C., and L.G. Eldredge, 1999. Nonindigenous Marine Species Introductions in the Harbors of the South and West Shores of Oʻahu, Hawaiʻi. Bishop Museum Technical Report No. 15.
Hawaiʻi Department of Land and Natural Resources. 2011. Commercial Marine Landings Summary Trend Report: Calendar Year 2011. 19 pages. https://dlnr.hawaii.gov/dar/files/2014/04/cmlstr2011.pdf
ISSG (Invasive Species Specialist Group), 2014. Ecology of Oreochromis mossambicus. Global Invasive Species Database.
https://www.issg.org/database/species/ecology.asp?si=131&fr=1&sts=sss
Updated URL: https://issg.org/database/species/ecology.asp?si=131&fr=1&sts=&%20ang=TC.
MacKenzie, R.A. and G.L. Bruland, 2012. Nekton Communities in Hawaiian Coastal Wetlands: The Distribution and Abundance of Introduced Fish Species.Estuaries and Coasts, 35:212–226.
Navy. 2007. Step 7 Baseline ERA, Pearl Harbor: Sediment Remedial Investigation.
Northcott, M.E., Beveridge, M.C.M. and L.G. Ross, 1991. A laboratory investigation of the filtration and ingestion rates of the tilapia, Oreochromis niloticus, feeding on two species of blue-green algae. Environmental Biology of Fishes, 31: 75-85.
Randall, J.E. 1987. Introductions of marine fishes to the Hawaiian Islands. Bulletin of Marine Science 41(2):490-502.
Russell , D.J., Thuesen, P.A., and F.E. Thomson, 2012. A review of the biology, ecology, distribution and control of Mozambique tilapia, Oreochromis mossambicus (Peters 1852) (Pisces: Cichlidae) with particular emphasis on invasive Australian populations. Rev Fish Biol Fisheries, 22:533–554.
Tetra Tech, Inc. 2009. Marine Resources Study Field Sampling Results and Risk Assessment, Mākua Military Reservation, Oʻahu, Hawaiʻi.
USGS,2013. Oreochromis mossambicus. USGS Nonindigenous Aquatic Species Database, Gainesville, FL. https://nas.er.usgs.gov/queries/factsheet.aspx?SpeciesID=466Revision Date:4/26/2013 Updated URL: https://nas.er.usgs.gov/queries/FactSheet.aspx?speciesID=466
Yang, F., Wilcox, B., Jin, S., Aguirre, A.A., Rougée, L., Xu, Y., and Y. Lu, 2008. Detection and quantitative analysis of polychlorinated biphenyls in tilapia from Hawaiian waters. Chemosphere, 73: 133–137.
Photo Credit: Blue tilapia (Oreochromis aureus) by Leonard Lovshin, US Army Corps of EngineersLicensed under Public domain via Wikimedia Commons – https://commons.wikimedia.org/wiki/File:Blue_tilapia_identification_ASACE.jpg
16 – Spectacled parrotfish (Chlorurus perspicillatus) and Yellowbar parrotfish (Calotomus zonarchus)
 Spectacled parrotfish (Chlorurus perspicillatus) Uhu Uliuli, Uhu ʻahuʻula |
 Yellowbar parrotfish (Calotomus zonarchus) ponuhunuhu |
Native Hawaiian Species: Both species are native to the Hawaiian Islands. The spectacled parrotfish also occurs at Johnston Atoll; otherwise, both species are endemic to Hawaiʻi (Bishop Museum 1997, Hawaiʻi DLNR 2005).
Habitat: Like all parrotfishes, these species are associated with coral reefs. Spectacled parrotfish occur in surface waters and range to more than 60 meters (200 feet) deep. The yellowbar parrotfish is not known from shallow surface waters, but occur in waters at least 10 meters (35 feet) deep (Hawaiʻi DLNR 2005).
Locations: The spectacled parrotfish is widely distributed across Hawaiʻi. The yellowbar parrotfish occurs from Oʻahu through the Northwestern Hawaiian Islands (Hawaiʻi DLNR 2005).
Cultural Use (historical and current): None reported.
Recreational Harvest: Both species are fished recreationally and are especially vulnerable to nighttime spearfishing (Hawaiʻi DLNR 2005).
Commercial Harvest: Parrotfishes are harvested commercially, although landings are not tracked by species. (DLNR 2011) reported that 72,000 pounds of uhu were landed in Hawaiʻi in that year.
Home Range: The mean home range for the redlip parrotfish (Scarus rubroviolaceus) ranges from 382 to 834 m2 at 5 m depth and 1,043 to 2,279 m2 at 15 m depth, depending on the phase of the fish.
Size/Body Weight: These parrotfishes typically grow to about 30 centimeters (1 foot) in total length in the MHI (Hawaiʻi DLNR 2005). Larger individuals have been reported from the NWHI. The size at which individuals become terminal phase males varies among islands, possibly in response to differential predation from sharks and other large piscivorous fishes such as trevallies (Family Carangidae). Terminal phase males on Midway were longer than 50 cm (about 22 inches) (DeMartini et al. 2005). No body weight data were found.
Diet/Ingestion Rates: Parrotfishes are herbivorous and graze algae from rock and coral surfaces (Hawaiʻi DLNR 2005). No food or sediment ingestion rates were found.
Predators: In the NWHI, apex predators such giant trevally (Caranx ignobilis) and bluefin trevally (C. melampygus), feed heavily on parrotfishes (DeMartini et al. 2005). In the MHI, where stocks of apex predators have been reduced by harvest, humans are the predominant predator on parrotfishes (Friedlander and DeMartini 2002).
Tissue Data (or other use in ecological or human health risk assessment)/Bioaccumulation factors: No tissue data or bioaccumulation factors were found.
Conservation Status: No special status (Russel et al. 2012)
References
Bishop Museum. 1997. Spectacled Parrotfish. https://www.bishopmuseum.org/research/natsci/fish/images/parrot.html
DeMartini, E.E., A.M. Friedlander, and S.R. Holzwarth. 2005. “Size at sex change in protogynous labroids, prey body size distributions, and apex predator densities at NW Hawaiian atolls.” Marine Ecology Progress Series, vol. 297, pp. 259–271.
Friedlander, A.M. and E.E. DeMartini. 2002. Contrasts in density, size, and biomass of reef fishes between the northwestern and the main Hawaiian islands: the effects of fishing down apex predators. Marine Ecology Progress Series 230: 253–264.
Hawaiʻi Department of Land and Natural Resources (DLNR), 2005. Marine Fishes: Spectacled parrotfish Uhu Chlorurus perspicillatus, Yellowbar parrotfish Uhu Calotomus zonarchus, Hawaiʻi’s Comprehensive Wildlife Conservation Strategy. https://dlnr.hawaii.gov/wildlife/files/2013/09/Fact-Sheet-parrotfishes.pdf
Hawaiʻi Department of Land and Natural Resources (DLNR). 2011. Commercial Marine Landings Summary Trend Report: Calendar Year 2011. 19 pages. https://dlnr.hawaii.gov/dar/files/2014/04/cmlstr2011.pdf
Russell, B., Choat, J.H., Clements, K.D., Rocha, L.A., Myers, R., Lazuardi, M.E., Muljadi, A., Pardede, S. & Rahardjo, P. 2012. Calotomus zonarchus. The IUCN Red List of Threatened Species. Version 2014.2. <www.iucnredlist.org>. Downloaded on 23 August 2014.
Photo Credit: Spectacled parrotfish, “Reef0620 – Flickr – NOAA Photo Library” by Dr. Dwayne Meadows, NOAA/NMFS/OPR. – NOAA Photo Library: reef0620. Licensed under Public domain via Wikimedia Commons – https://commons.wikimedia.org/wiki/File:Reef0620_-_Flickr_-_NOAA_Photo_Library.jpg#mediaviewer
17 – Moray eel (Muraenidae)
 Moray eel (Muraenidae) |
Native Hawaiian Species: Several species are native to Hawaiʻi. Forty-two species of moray eel have been found in the Hawaiian Islands (Böhlke and Randall, 2000). Common species include the yellow margin moray (G. flavimarginatus) and undulated moray (G. undulates). Some species of moray eel are endemic to the Hawaiian Islands, including the Atoll moray (Gymnothorax atolli), Nutting’s moray (G. nuttingi), brown speckled eel (G. steindachneri), and possibly the manyvertebrate moray (G. polyspondylus) (Böhlke and Randall, 2000).
Habitat: Moray eels occur in shallow to moderately deep tropical and subtropical seas. They are frequently observed in coral reefs or lagoons. They tend to hide in reefs or rocky bottoms (Böhlke and Randall 2000).
Locations: Throughout the Hawaiian Islands.
Seasonality (year-round resident or migrant): Year-round resident.
Cultural Use (historical and current): None identified.
Recreational Harvest: Moray eels are harvested and consumed throughout the Indo-Pacific region, including the Hawaiian Islands. However ciguatera poisoning may result from eating large piscivorous reef fishes, such as large morays (greater than 4 kg), particularly the yellow margin moray, undulated moray, giant moray (G. javanicus), and white mouth moray (G . meleagris) (Böhlke and Randall, 2000).
Commercial Harvest: Some species may be harvested.
Home Range: The yellow margin moray and undulated moray occupy reefs and rocky substrates from depths of 1 to 150 meters (Reece et al. 2011). Other morays have a more restricted range. For example, the snowflake moray (Echidna nebulosa) and zebra moray (Gymnomuraena zebra) occur between 0 and 15 meters but are most common at less than 2 meters (Hiatt & Strasburg 1960, Yukihira et al. 1994 [as cited in Reece et al. 2011]). The average depths where eels were captured for a study across the Indian Ocean and Pacific Ocean were 22 meters for the yellow margin moray, 24 meters for the undulated moray, 1.9 meters for the zebra moray, and 1.8 meters for the snowflake moray (Reece et al. 2011).
Size/Body Weight: The yellow margin moray and undulated moray are large fish. The undulated moray ranged from 312 to 628 mm (females) and 282 to 756 mm (males) (Böhlke and Randall 2000). The yellow margin moray ranged from 230 to 1175 mm. The zebra moray ranged from 427 to 920 mm (females) and 425 to 734 mm (males). All three of these species may reach 1500 mm. The snowflake moray ranged from 273 to 570 mm (females) and 302 to 703 (males), with a maximum length of 750 mm (Böhlke and Randall 2000).
Diet/Ingestion Rates: Moray eels in Hawaiʻi are generally separated into two groups based on their diet. The species with fang-like teeth, including the yellow margin moray and undulated moray, feed on fishes and soft-bodied invertebrates (e.g., octopus). The species with pebble-like teeth, such as the snowflake moray and zebra moray, feed on crustaceans (e.g., crabs and molluscs) (Waikīkī Aquarium 2014).
Predators: Humans and large fish species (e.g., grouper)
Tissue Data (or other use in ecological or human health risk assessment)/Bioaccumulation Factors: The conger eel (Conger cinereus), undulated moray eel (Gymnothorax undulates), whitemouth moray eel (Gymnothorax meleagris), and yellowmargin moray eel (Gymnothorax flavimarginatus) collected from French Frigate Shoals were analyzed for PCBs and metals (arsenic, cadmium, chromium, copper, lead, mercury, selenium, and zinc) (Miao et al. 2000, Miao et al. 2001). The results indicated that the undulated moray eel bioaccumulated high levels of arsenic compared with the other eel species (Miao et al. 2001). The other metal concentrations varied little among species.
Conservation Status: Not threatened.
References
Bohlke, E.B. and J.E. Randall, 2000. A Review of the Moray Eels (Angulliformes: Muraenidae) of the Hawaiian Islands, with Descriptions of Two New Species. Proceedings of the Academy of Natural Sciences of Philadelphia, 150: 203-278.
Miao, X.S., Swenson, C., Woodward, L.A., and Q.X. Li, 2000. Distribution of polychlorinated biphenyls in marine species from French Frigate Shoals, North Pacific Ocean. The Science of the Total Environment, 257: 17-28.
Miao, X.S., Woodward, L.A., Swenson, C., and Q.X. Li, 2001. Comparative Concentrations of Metals in Marine Species from French Frigate Shoals, North Pacific Ocean. Marine Pollution Bulletin, 42 (11): 1049-1054.
Reece, J.S., Bowen, B.W., Smith, D.G., and A. Larson, 2011. Comparative phylogeography of four Indo-Pacific moray eel species (Muraenidae) reveals comparable ocean-wide genetic connectivity despite five-fold differences in available adult habitat. Marine Ecology Progress Series, 437: 269–277.
Waikīkī Aquarium, 2014. Undulated Moray. Animal Guide. https://www.waikikiaquarium.org/experience/animal-guide/fishes/eels/undulated-moray/ Accessed August 27, 2014.
Photo Credit: Jon Hanson Licensed under Public domain via Wikimedia Commons – https://commons.wikimedia.org/wiki/File:Yellow_Margined_Moray_Eel.jpg#mediaviewer
18 – Wedge-tailed shearwater (Puffinus pacificus)
 Wedge-tailed shearwater (Puffinus pacificus) |
Native Hawaiian Species: Yes
Habitat: Occurs over the open ocean most of the year. Nests in burrows, which may be concealed or in the open. Shearwater burrows at Mālaekahana ranged from concealment under thickets of naupaka, at the base of ironwood and heliotrope trees, to bare sand under clumps of ʻakiʻaki grass (Smith et al. 2002).
Locations: This bird occurs throughout most of the tropical and subtropical Pacific and Indian Oceans, including the Hawaiian Islands (Harrison 1983, Harrison 1990, Whittow 1997 [as cited in Smith et al. 2002). It is a common breeder on almost every island in Hawaiʻi and on many small offshore islets (Pyle and Pyle 2009).
Seasonality (year-round resident or migrant): Migrant. The wedge-tailed shearwater spends much of the year on the open ocean but returns to coastal areas to breed (Smith et al. 2002). It returns to nesting areas on Oʻahu in April (Smith et al. 2002). In November, the fledglings fly out to sea (Smith et al. 2002).
Cultural Use (historical and current): None identified.
Recreational Harvest: None.
Commercial Harvest: None. Seabirds in Hawaiʻi are protected by the federal Migratory Bird Treaty Act (50 CFR 10) and by State law under Title 13, Part 2, Chapter 125 of the Hawaiʻi Administrative Rules, which prohibit the hunting, capturing, killing, possession, shipping, etc., of migratory birds, unless authorized by a permit (Smith et al. 2002).
Home Range: Not identified. It remains close to its burrow during nesting.
Size/Body Weight: The wedge-tailed shearwater was reported to weigh from 0.30 to 0.57 kg (sample size = 576 birds) (Bull, 2006).
Diet/Ingestion Rates: An adult shearwater consumes an average 35.01 ± 1.34 grams per day, based on a study in Australia (Peck and Congdon 2005 [as cited in McDuie et al. 2013]). A study in the NWHI reported that fish comprised 67.0% and cephalopods 28.6% by volume of stomach contents. By number of items, fish comprised 73.3% and cephalopods 23.1%. The remaining small portions consisted of crustaceans, insects, and coelenterates. Typical prey size was approximately 5.7 cm in length. Common fish prey include Mullidae and Carangidae, especially shortfin scad (Decapterus macrosoma); cephalopods were mostly Ommastrephidae (Marchant and Higgins 1990 [as cited in Australian Department of the Environment 2014]).
Predators: Humans and introduced mammals, such as dogs, cats, mongooses, and rats (Olson and James 1982, Steadman 1995 [as cited in Smith et al. 2002]). Predation by feral cats was reported to reduce reproductive success to near zero at Mālaekahana, and pedestrian traffic can collapse nesting burrows (Smith et al. 2002). Invasive ants were observed to injure nesting shearwaters on windward Oʻahu (Plentovich et al. 2009).
Tissue Data (or other use in ecological or human health risk assessment)/Bioaccumulation Factors:
- Feathers from wedge-tailed shearwaters from Midway Atoll were analyzed for metals (arsenic, cadmium, chromium, manganese, mercury, lead, selenium, and tin) (Burger and Gochfeld 2000). Chromium was high in the wedge-tailed shearwaters compared to other sea birds.
- Feathers from flesh-footed shearwaters (Puffinus carneipes) from New Zealand, Lord Howe Island, and Australia were analyzed for metals (Bond and Lavers 2011). Mercury, and potentially arsenic and cadmium were noted as toxicological concerns for the species.
Conservation Status: Not threatened. Another species, Newell Shearwater (Puffinus newelli) is threatened.
References
Australian Department of the Environment, 2014. Ardenna pacifica in Species Profile and Threats Database, Department of the Environment, Canberra. https://www.environment.gov.au/sprat. Accessed August 26, 2014.
Bond, A.L. and J.L. Lavers, 2011. Trace Element Concentrations in Feathers of Flesh-footed Shearwaters (Puffinus carneipes) from Across Their Breeding Range. Arch Environ Contam Toxicol,61: 318–326.
Bull, L.S. 2006. Influence of migratory behaviour on the morphology and breeding biology of Puffinus shearwaters. Marine Ornithology, 34: 25–31.
Burger, J. and M. Gochfeld, 2000. Metal levels in feathers of 12 species of seabirds from Midway Atoll in the northern Pacific Ocean. The Science of the Total Environment, 257: 37-52.
McDuie, F., Goulding, W., Peck, D.R, and B.C. Congdon, 2013. Divergence in chick developmental patterns among wedge-tailed shearwater populations. Marine Ecology Progress Series, 485: 275–285.
Plentovich, S., Hebshi, A. and S. Conant, 2009. Detrimental effects of two widespread invasive ant species on weight and survival of colonial nesting seabirds in the Hawaiian Islands. Biol Invasions, 11:289–298.
Pyle, R.L., and P. Pyle. 2009. The Birds of the Hawaiian Islands: Occurrence, History, Distribution, and Status.B.P. Bishop Museum, Honolulu, HI, U.S.A. Version 1 (31 December 2009) https://hbs.bishopmuseum.org/birds/rlp-monograph/
Smith, D.G., Polhemus, J.T., and E.A. VanderWerf, 2002. Comparison of Managed and Unmanaged Wedge-Tailed Shearwater Colonies on Oʻahu: Effects of Predation. Pacific Science, 56(4): 451-457.
Photo Credit: Duncan Wright, USFWS Licensed under Public domain via Wikimedia Commons – https://commons.wikimedia.org/wiki/File:W-tail_with_chick.jpg#mediaviewer
19 – Black-crowned night heron (Nycticorax nycticorax hoactli)
 Black-crowned night heron (Nycticorax nycticorax hoactli) |
Native Hawaiian Species: yes
Habitat: The night heron is frequently found in permanent freshwater wetlands, lowland streams, and man-made wetlands. It is also known to occur in lagoons, reefs, and lava benches exposed during low tide (Engilis and Pratt 1993).
Locations: It occurs on every continent except Australia and Antarctica (U.S. Army Center for Health Promotion and Preventive Medicine 2004). Within the Hawaiian Islands, it is observed commonly on Kauaʻi, Oʻahu, Molokaʻi, Maui, and Hawaiʻi and reported less often on Niʻihau, Lānaʻi, and Kahoʻolawe (Pyle and Pyle 2009).
Seasonality (year-round resident or migrant): Year-round resident.
Cultural Use (historical and current): None identified.
Recreational Harvest: None.
Commercial Harvest: None.
Home Range: Most colonies of the black-crowned night heron are in the vicinity of large wetlands. During nesting, both the male and female defend a territory around a large nest (approximately 8 ft wide and 4 ft high. Adults often return to previously used nests (Davis 1993 [as cited in U.S. Army Center for Health Promotion and Preventive Medicine 2004]; Custer et al. 1980 [as cited in USGS 2010]). Individuals disperse widely after nesting is completed.
Size/Body Weight: Adult black-crowned night herons are approximately 58 to 65 cm in length, with an average mass of 883 grams (Dunning 1993 [as cited in USGS 2010]). Body mass in four males ranged from 785 to 1014 grams with an average mass of 913 grams; four females ranged from 727 to 862 grams with an average mass of 827 grams (Gross 1923 [as cited in U.S. Army Center for Health Promotion and Preventive Medicine 2004])
Diet/Ingestion Rates: While fish are the primary food source, the black-crowned night heron is an opportunistic feeder (Davis 1993 [as cited in U.S. Army Center for Health Promotion and Preventive Medicine, 2004]). Its diet varies by habitat and regional, but is typically about 50 percent fish, 40 percent insects and crustaceans, and 10 percent amphibians and reptiles (Palmer 1962 [as cited in Madenjian and Gabrey 1995]). The black-crowned night heron may prey upon Hawaiian coot chicks (Brisbin et al. 2002 [as cited in USFWS 2011]). This heron may consume fish at a rate of 0.15 kg/day (Alberta Agriculture and Rural Development 1999). A food consumption rate of 0.061 kg/kg body weight/day was estimated using a mean body weight of 0.87 kg and assuming a diet of 59 percent fish, 37 percent invertebrates, 7 percent amphibians, and 1.7 percent mammals with respective water contents of 75, 76, 85, and 68 percent. Using the same body weight, a water ingestion rate of 0.062 L/kg BW/day was calculated (U.S. Army Center for Health Promotion and Preventive Medicine 2004). The heron may ingest soil or sediment when feeding on burrowing crustaceans.U.S. Army Center for (Health Promotion and Preventive Medicine 2004) estimated that a soil/sediment ingestion rate of 2 percent, as reported in (Beyer et al. 1994), was appropriate.
Predators: Not identified.
Tissue Data (or other use in ecological or human health risk assessment)/Bioaccumulation Factors:
- See Pearl Harbor BERA for use of black-crowned night heron as assessment endpoint (Navy 2007b).
Conservation Status: Not threatened.
References
Alberta Agriculture and Rural Development, 1999. Predator Damage Control in Cultured Fish. Agdex 485/685-1. December. https://www1.agric.gov.ab.ca/$department/deptdocs.nsf/all/agdex821 Updated URL: https://open.alberta.ca/publications/2377140
Engilis, A. and T.K. Pratt, 1993. Status and Population Trends of Hawaiʻi’s Native Waterbirds, 1977-1987. The Wilson Bulletin, 105 (1): 142-158.
Madenjian, C.P. and S.W. Gabrey, 1995. Waterbird Predation on Fish in Western Lake Erie: A Bioenergetics Model Application. The Condor, 97(1): 141-153.
Navy. 2007. Step 7 Baseline ERA, Pearl Harbor: Sediment Remedial Investigation.
Pyle, R.L., and P. Pyle. 2009. The Birds of the Hawaiian Islands: Occurrence, History, Distribution, and Status.B.P. Bishop Museum, Honolulu, HI, U.S.A. Version 1 (31 December 2009) https://hbs.bishopmuseum.org/birds/rlp-monograph/
U.S. Army Center for Health Promotion and Preventive Medicine, 2004. Development of Terrestrial Exposure and Bioaccumulation Information for the Army Risk Assessment Modeling System (ARAMS). April. https://el.erdc.usace.army.mil/arams/pdfs/usachppm.pdf Updated URL: https://denix.osd.mil/cmrmp/chemicalmanagement/riskassessment/general-information/wildlife-exposure/
USFWS (U.S. Fish and Wildlife Service), 2011. Recovery Plan for Hawaiian Waterbirds, Second Revision. https://www.fws.gov/pacificislands/CH_Rules/Hawaiian%20Waterbirds%20RP%202nd%20Revision.pdf
USGS, 2010. Biological and Ecotoxicological Characteristics of Terrestrial Vertebrate Species. Species Reports. https://www.pwrc.usgs.gov/contaminants-online/
Photo Credit: “Black-crowned night heron. ʻAukuʻu. Nycticorax nycticorax hoactii” Photo taken by Tom Fake, National Park Service.
20 – Hawaiian coot (Fulica alai)
 Hawaiian coot (Fulica alai) ʻAlae Keʻokeʻo |
Native Hawaiian Species: Yes
Habitat: Low elevations in wetland habitats with both emergent plant growth and open water (USFWS 2011). The coot prefers freshwater wetlands but will use brackish wetlands.
Locations: Currently on all of the main Hawaiian Islands except Kahoʻolawe, which lacks suitable wetland habitat (USFWS 2011). It is present on Lānaʻi in artificial wetland areas near water treatment sites (USFWS 2011).
Seasonality (year-round resident or migrant): Year-round resident (USFWS 2011).
Cultural Use (historical and current): None identified.
Recreational Harvest: Hunted in early twentieth century, but now protected (Reed et al. 2011).
Commercial Harvest: None.
Home Range: The coot typically feeds and nests in the same area but will travel long distances when food is not locally available (Shallenberger 1977 [as cited in USFWS 2011]).
Size/Body Weight: Adult Hawaiian coots weigh approximately 0.53 ± .08 kilograms (n = 231) (Desrochers et al. 2010). The Pearl Harbor BERA used an adult body weight of 0.56 kg (Navy 2007b).
Diet/Ingestion Rates: The coot forages near the surface of the water or deeper; it will dive for food and forage in submerged mud or sand. It also grazes on upland grassy sites, such as golf courses adjacent to wetland (USFWS 2011). Food items include aquatic plants, particularly seeds and leaves; invertebrates such as snails, crustaceans, and insects; and small fish (Schwartz and Schwartz 1949 [as cited in USFWS 2011]).
No food or sediment ingestion rates were found. A large-scale study of the American coot, a closely related species (formerly considered another subspecies) common on the U.S. mainland, reported that the stomach held up to 30 percent sand or sediment (Jones 1940). The Pearl Harbor BERA calculated a normalized food ingestion rate of 0.071 kg food/kg body weight-day) using methods in (EPA 1993) and an incidental sediment ingestion rate of 3 percent for the coot (Navy 2007b).
Predators: Non-native cats, rats, mongooses, dogs, and to a lesser extent wild pigs, barn owls, cattle egrets, predatory fish, and bullfrogs prey on eggs, young, or adult birds (Underwood et al. 2013). The native black-crowned night heron may prey upon Hawaiian coot chicks (Brisbin et al. 2002 [as cited in USFWS 2011]).
Tissue Data (or other use in ecological or human health risk assessment)/Bioaccumulation factors: See Pearl Harbor BERA for use of Hawaiian coot as assessment endpoint (Navy 2007b).
Waiākea Pond (Hilo) ERA provides life history parameters for food chain modeling and toxicity reference values for arsenic.
Conservation Status: Endangered. The main cause of decline of the Hawaiian coot is loss of wetland habitat (USFWS 2011).
References
Desrochers, D.W., Mcwilliams, S.R. , and J.M. Reed, 2010. Evaluating if Energy and Protein Limit Abundance of Hawaiian Moorhen. Journal of Wildlife Management, 74(4):788-795.
Jones, J.C. (1940). Food Habits of the American Coot with Notes on Distribution. Wildlife Research Bulletin 2, U.S. Department of the Interior, Bureau of Biological Survey: 52.
Navy. 2007. Step 7 Baseline ERA, Pearl Harbor: Sediment Remedial Investigation.
Reed, J.M., C.S. Elphick, E.N. Ieno, and A.F. Zuur, 2011. Long-term population trends of endangered Hawaiian waterbirds. Population Ecology 53:473–481.
The Environmental Company, Inc. 2005 Ecological Risk Assessment, Waiākea Pond, Hilo, Hawaiʻi.
U.S. Environmental Protection Agency (EPA) (1993). Wildlife Exposure Factors Handbook. Appendix: Literature Review Database. Washington, D.C., Office of Research and Development. II of II.
U.S. Fish and Wildlife Service (FWS). 2011. Recovery Plan for Hawaiian Waterbirds, Second Revision. https://www.fws.gov/pacificislands/CH_Rules/Hawaiian%20Waterbirds%20RP%202nd%20Revision.pdf
Underwood, J.G., M. Silbernagle, M. Nishimoto, and K. Uyehara. 2013. Managing Conservation Reliant Species: Hawaiʻi’s Endangered Endemic Waterbirds. PLoS ONE 8(6): e67872.
Photo Credit: “Hawaiian coot. ʻAlae keʻokeʻo. Fulica alai” Photo taken by Bryan Harry, National Park Service. https://www.botany.hawaii.edu/basch/uhnpscesu/htms/kahobird/fish_pops/Rallidae/rail01.htm
21 – Green sea turtle (Chelonia mydas)
 Green sea turtle (Chelonia mydas) Honu |
Native Hawaiian Species: yes
Habitat: The green sea turtle forages in seagrass beds, shallow patch reefs, and coral-covered areas; it rests on deep mud bottom channels and in small caves and crevices in the sides of reefs (Brill et al. 1995). Immature green turtles stay in the open ocean, moving inshore as adults (Parker et al. 2011).
Locations: The green sea turtle occurs in tropical waters worldwide and is the most common sea turtle in Hawaiʻi, occurring throughout the islands. Most nesting is at French Frigate Shoals (Balazs 1980).
Seasonality (year-round resident or migrant): Year-round resident
Cultural Use (historical and current): Historically, the green sea turtle was used as a food source and harvest was often restricted to special occasions. Certain parts of the green turtle, such as the fat, were also used for medicinal purposes. In addition, parts of the turtle were used as containers, tools, and ornaments (NOAA 1998).
Recreational Harvest: Sea turtles and their eggs are harvested for food (NOAA, 1998).
Commercial Harvest: Incidental harvest by commercial fisheries (NOAA, 1998).
Home Range: Telemetry tracing the journeys of male and female turtles between breeding and foraging areas found some individuals traveled 1050 to 1200 km (Balazs and Ellis, undated). Adults feed in areas less than 10 meters deep, typically only 3 meters deep (Balazs 1980).
Size/Body Weight: The straight carapace length of the green sea turtle turtles is <65 cm for juveniles; 65-81 cm for sub-adults, and >81 cm for adults. The mean body weight of adult females is 110 kg (range 68-148 kg) based on a sample size of 69 turtles (Balazs 1980).
Diet/Ingestion Rates: Algae is the dominant food, supplemented by jellyfish, salps, mollusks, sponges, and tubeworms (NOAA 2018). An adult green sea turtle consumes the equivalent of only 0.24 to 0.33 % of its body weight each day (dry weight to wet weight ratio) (Bjorndal 1980). Juvenile turtles in the pelagic phase (which lasts from 5 to 10 years) are carnivorous or omnivorous, consuming zooplankton, pelagic crustaceans, and mollusks (Parker et al. 2011).
Predators: Sea turtle eggs are eaten by feral dogs and cats, rats, mongooses, and ghost crabs. Hatchlings may be taken by large crabs and fishes. Humans and tiger sharks prey on juvenile and adult turtles (Balazs 1980, NOAA 2018).
Tissue Data (or other use in ecological or human health risk assessment)/Bioaccumulation Factors:
- Arsenic concentrations were measured in green turtle tissues (liver, kidney, muscle, and stomach contents) collected in Japan (Agusa et al. 2008). Concentrations of arsenic in the stomach versus tissues were reported to indicate bioaccumulation of arsenic.
- Toxicity reference values were derived for sea turtles on Tern Island using terrestrial bird laboratory toxicological studies (USCG 2000).
Conservation Status: Threatened; Hawaiian population is under review for delisting (Federal Register, 2012).
References
Agusa, T., Takagi, K., Iwata, H. and S. Tanabe, 2008. Arsenic species and their accumulation features in green turtles (Chelonia mydas). Marine Pollution Bulletin, 57: 782–789.
Balazs, G.H. 1980. Synopsis of Biological Data on the Green Turtle in the Hawaiian Islands. NOAA Technical Memorandum. October. NOAA-TM-NMFS-SWFC-7. https://noaa.ntis.gov/view.php?pid=NOAA:ocm07369432 Updated URL: https://repository.library.noaa.gov/view/noaa/5404/noaa_5404_DS1.pdf
Balazs, G.H and D.M. Ellis. Satellite telemetry of migrant turtles breeding in the Hawaiian Islands. https://www.turtles.org/ffs/migrate/ffsmigrt.htm
Bjorndal, K.A., 1980. Nutrition and Grazing Behavior of the Green Turtle Chelonia mydas. Marine Biology, 56: 147-154.
Brill, R.W., Balazs, G.H., Holland, K.N., Chang, R.K.C., Sullivan, S., George, J.C., 1995. Daily Movements, Habitat Use, and Submergence Intervals of Normal and Tumor-Bearing Juvenile Green Turtles (Chelonia mydas L.) within a Foraging Area in the Hawaiian-Islands. Journal of Experimental Marine Biology and Ecology, 185: 203-218.
Federal Register, 2012. Endangered and Threaten Wildlife; 90-Day Finding on a Petition to Delist the Green Turtle in Hawaiʻi and Notice of Status Review. Volume 77, Number 148. August 1, 2012. p. 45571 https://www.nmfs.noaa.gov/pr/species/frnotices/delisting/green_turtle_Hawaiʻi_90d_77fr45571.pdf
NOAA, 1998. Recovery Plan for U.S. Pacific Populations of the Green Turtle (Chelonia mydas). https://www.fpir.noaa.gov/Library/PRD/Sea%20Turtles/turtle_green_pacific_recoveryplan.pdf
NOAA, 2018. NOAA Fisheries Pacific Island Regional Office. Website. https://www.fpir.noaa.gov/PRD/prd_green_sea_turtle.html Accessed October 2018.
Parker, D.M., Dutton, P.H., and G.H. Balazs, 2011. Oceanic Diet and Distribution of Haplotypes for the Green Turtle, Chelonia mydas, in the Central North Pacific. Pacific Science, 65(4):419-431.
U.S. Coast Guard (2000). Tern Island Ecological Risk Assessment.
Photo Credit: Andy Bruckner, NOAA (https://www.nmfs.noaa.gov/pr/species/turtles/photos.htm)
22 – Monk seal (Monachus schauinslandi)
 Monk seal (Monachus schauinslandi) ʻīlio-holo-i-ka-uaua or na mea hulu |
Native Hawaiian Species: Yes. Endemic to Hawaiʻi (NOAA 2018)
Habitat: The Hawaiian monk seal uses shallow water reef habitat for pupping, weaning and foraging, sandy beach areas for resting, and deeper reef areas for foraging (Kittinger et al. 2011). It forages in various habitats such as coral reefs, sandy bottom, rubble flats, and sub-photic slopes (Sprague et al. 2013). Most foraging dives are 200 meters or less (Sprague et al. 2013).
Locations: The largest population of Hawaiian monk seal occurs in the NWHI (approximately 1,400); a second population of about 200 seals occurs on the MHI (Sprague et al. 2013). The NWHI population is declining, while the number of seals in the MHI is increasing (Lopez et al. 2012, Johanos et al. 2014).
Seasonality (year-round resident or migrant): Year-round resident; some movement between the two populations occurs.
Cultural Use (historical and current): The Hawaiian monk seal is seen by some native Hawaiians as an interloper from the NWHI that competes with local subsistence fishing communities. Other people report strong positive cultural associations and a feeling of stewardship toward the seal (Kittinger et al. 2011).
Recreational Harvest: Monk seals may have been harvested historically for meat and fur but harvest is illegal now under the Endangered Species Act (Kittinger et al. 2011).
Commercial Harvest: The monk seal was hunted historically but is now protected from harvest.
Home Range: The species ranges over 2500 km throughout the Hawaiian Islands (Baker et al. 2004, 2012). Although individual monk seals may move between the NWHI and the MHI, most do not. Pups tend to stay on their natal island, but adults may visit adjacent islands throughout the year (Johanos et al. 2014). Foraging trips may extend to 322 km from the island of origin (Stewart 2004, Stewart et al. 2006 [as cited in Johanos et al. 2014]).
Size/Body Weight: The average body mass for monk seals is 170 kg for an adult (>5 years), 140 kg for a subadult (3-5 years), and 66 kg for a juvenile (weaning to 3 years) (Sprague et al. 2013).
Diet/Ingestion Rates: The Hawaiian monk seal consumes more than 22 families of fish and marine invertebrates. Principal prey items include triggerfish, moray and white eels, large crustaceans, and surgeonfishes (Sprague et al. 2013). Generally, the monk seal does not eat apex predators such as tuna or mahi, preferring the slower reef fishes that are easier to catch (Baker et al. 2012).
| Life Stage | Daily food ingestion (kg/day)1 | Food Ingestion Rate (% body mass/day)1 | Sediment Ingestion (grams/day)2 |
| Adult | 5.89 | 3.5 | 3 to 10 |
| Subadult | 7.61 | 5.5 | No data |
| Juvenile | 5.01 | 7.6 | No data |
| Nursing Pup | No data | No data | 10 (for 5 weeks) |
| Source: 1 Sprague et al. 2013 2 Woodward-Clyde Consultants 1994. |
|||
Predators: Monk seal pups may be taken by sharks or injured by adult male seals (Baker et al. 2012).
Tissue Data/Bioaccumulation Factors:
Mean concentrations of persistent organic pollutants (POP) in monk seal blubber were similar in seals in the Main Hawaiian Islands and the Northwestern Hawaiian Islands; however, some individuals from the main Hawaiian Islands had high contaminant levels (Lopez et al. 2012). Adult males generally have higher blubber and serum concentrations of POPs than do adult females or juveniles (Ylitalo et al. 2008; Lopez et al. 2012). POPs in monk seals from NWHI were generally equal to or lower than those reported for other pinniped species in the North Pacific Ocean (Ylitalo et al. 2008). Monk seals from French Frigate Shoals were analyzed for PCBs, DDT and DDT metabolites (Willcox et al. 2004). Correlations were noted between concentrations of DDE and PCBs in blubber or blood and body mass, age, or condition (Willcox et al. 2004).
Blood and Blubber Concentrations: Organochlorine Compounds (DDTs and dioxin-like PCBs): Health effect levels and reference levels in North Pacific Ocean; safe upper PCB concentration of 8700 ng/g, lw for marine mammal blood; 17,000 ng/g, lw for PCBs in blubber
Sediment Preliminary Remediation Goals (PRG) protective of monk seal:
Lead: 270 to 890 mg/kg lead at Kure Atoll (NWHI) (Woodward-Clyde Consultants 1994)
PCBs: 250 to 849 mg/kg in sand on the island at Kure Atoll (NWHI) (Woodward-Clyde Consultants 1994)
PCBs: 3 mg/kg PCB soil cleanup level, based on target action level of 0.165 mg/kg in sediment. Tern Island, French Frigate Shoals (NWHI) (USCG 2000).
Toxicity Reference Value (no effect daily dose) for monk seal:
Lead = 0.02 mg/kg-body weight/day at Kure Atoll (NWHI) (Woodward-Clyde Consultants 1994)
PCBs = 0.0190 mg/kg-body weight/day at Kure Atoll (NWHI) (Woodward-Clyde Consultants 1994)
Conservation Status: Federally Endangered
References
Baker, J.D., Howell, E.A., and J.J. Polovina, 2012. Relative influence of climate variability and direct anthropogenic impact on a sub-tropical Pacific top predator, the Hawaiian monk seal. Marine Ecology Progress Series 469: 175–189.
Baker, J.D. and T.C. Johanos, 2004. Abundance of the Hawaiian monk seal in the main Hawaiian Islands. Biological Conservation 116: 103–110.
Johanos, T.C., Harting, A.L., Wurth, T.A. and J.D. Baker, et al., 2014. Range-wide movement patterns of Hawaiian monk seals. Marine Mammal Science 30(3): 1165–1174.
Kittinger, J.N., Bambico, T.M., Watson, T.K., and E. W Glazier, 2011. Historical and contemporary significance of the endangered Hawaiian monk seal in Native Hawaiian culture. A report prepared for the NOAA Pacific Islands Regional Office. Impact Assessment, Inc., Honolulu. https://www.fpir.noaa.gov/Library/PRD/Hawaiian%20monk%20seal/MonkSeal_Report_IAI_Final.pdf
Lopez, J., D. Boyd, G.M. Ylitalo, C. Littnan and R. Pearce. 2012. “Persistent organic pollutants in the endangered Hawaiian monk seal (Monachus schauinslandi) from the main Hawaiian Islands.” Marine Pollution Bulletin 64(11): 2588-2598.
NOAA, 2018. NOAA Fisheries Pacific Island Regional Office. Website. https://www.fpir.noaa.gov/PRD/prd_hms_index.html Accessed October 2018.
Sprague, R., Littnan, C., and J. Walters, 2013. Estimation of Hawaiian monk seal consumption in relation to ecosystem biomass and overlap with fisheries in the main Hawaiian Islands. NOAA-TM-NMFS-PIFSC-37, 42 p. + Appendices. https://www.pifsc.noaa.gov/library/pubs/tech/NOAA_Tech_Memo_PIFSC_37.pdf Updated URL: https://apps-pifsc.fisheries.noaa.gov/library/pubs/tech/NOAA_Tech_Memo_PIFSC_37.pdf
USCG. 2000. Final Ecological Risk Assessment: Former U.S. Coast Guard LORAN Station, Tern Island, French Frigate Shoals, Hawaiʻi.
Willcox, M.K., Woodward, L.A., Ylitalo, G.M., Buzitis, J. Atkinson, S., and Q.X. Lin, 2004. Organochlorines in the free-ranging Hawaiian monk seal (Monachus schauinslandi) from French Frigate Shoals, North Pacific Ocean. Science of the Total Environment 322: 81–93.
Woodward-Clyde Consultants (WWC). 1994. Kure Atoll Scrap Metal Dump Ecological Risk Assessment. Final Report to U.S. Coast Guard. 248 pages. January.
Ylitalo, G.M., M. Myers, et al. 2008. “Organochlorine Contaminants in Endangered Hawaiian Monk Seals From Four Subpopulations in the Northwestern Hawaiian Islands.” Marine Pollution Bulletin 56(2): 231-244.
Photo Credit: “Monachus schauinslandi. Hawaiian monk seal” Photo taken by Tom Fake, U.S. National Park Service. https://www.botany.hawaii.edu/basch/uhnpscesu/htms/kahomamm/fish_pops/phocidae/seal02.htm