Appendix 21-F
Background/Ambient/Reference Concentrations Evaluating Bioavailability
Background/Ambient/Reference Concentrations
See Subsection 21.3.5.1. Additional information on background concentrations is being compiled by the HEER Office, and this information will be made available in the future.
Evaluating Bioavailability
In the initial steps of the SLERA, it is assumed that chemicals are 100 percent bioavailable. Refinement of this conservative assumption requires site-specific information. Total organic carbon (TOC) and grain size distribution data should be collected during the site investigation, so the potential bioavailability of the chemicals can be evaluated in the refinement step (Step 3a). These parameters should also be evaluated during the background comparison to determine whether the background sediment has similar physical characteristics as the site sediment because TOC and grain size can influence the amount of chemicals accumulating in sediment, as discussed below.
Very little debate remains over the role of grain size in influencing bioavailability. In general, finer-grained sediments have a proportionately higher number of binding sites (based strictly on increased surface area) than coarser-grained sediments. When fine-grained particles are ingested by an organism, a relatively higher dose of the chemical is also ingested.
The other hand, there are conflicting views on whether toxicity to sediment invertebrates is correlated with TOC. The tendency of PCBs, PAHs, and other organic chemicals (including mercury) to sorb to the organic carbon fraction is well documented and is used in equilibrium partitioning models to predict bioavailable fractions. However, the relationship between TOC and concentration of organic compounds is not as straightforward as the EqP models assume. For example, some studies have concluded that TOC normalization had little if any influence on the outcome of the screening process using the low sediment quality guidelines (McCready et al. 2006). Not all organic carbon is equally capable of binding organic chemicals; the type of organic carbon (humic matter particles, humic matter sorbed on mineral surfaces, animal and plant matter, combustion by-products) may also determine the strength of the association with organic compounds (Ghosh et al. 2003). Remediation engineers take advantage of the tendency of organic carbon to bind PCBs and apply specific types of activated organic carbon to sediments contaminated with PCBs, PCDD/Fs, and mercury as a means of reducing the bioavailability of chemicals to organisms (Millward Et al. 2005; Patmont et al. 2015; Gilmour et al. 2013).
It is recommended that TOC and grain size be analyzed in sediment ERAs so that the relationship can be independently tested in Hawaiʻi. If similar results are confirmed over time, this requirement may be modified or eliminated. Additional discussion of methods for measuring and interpreting results of grain size and TOC analyses is available in (Opel et al. 2011);
Under some conditions, chemical analysis of field-collected organisms can provide site-specific evidence of bioavailability of chemicals in sediment. Organisms should be resident at the site, relatively sessile, and exposed to sediment either through direct contact or ingestion of sediment (or both). Note that tissue concentrations will reflect exposure to chemicals in overlying water as well as sediment, potentially confounding data interpretation.
If tissue data are not available, chemical concentrations in food items can be calculated using sediment-to-fish and sediment-to-invertebrate BSAFs, as follows:
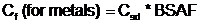
- Where:
- Cf = Contaminant concentration in food (mg/kg)
- Csd = Contaminant concentration in sediment (mg/kg)
- BSAF = Biota-sediment bioaccumulation factor (unitless)
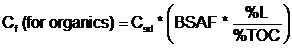
- Where:
- Cf = Contaminant concentration in food (mg/kg)
- Csd = Contaminant concentration in sediment (mg/kg)
- BSAF = Biota-sediment bioaccumulation factor (for organics) (unitless)
- %L = Percent lipids [species-specific value (dry weight)
- %TOC = Percent total organic carbon (site-specific value)
For the SLERA, conservative exposure assumptions should be used for food chain models, such as:
- Maximum sediment concentrations
- Conservative receptor body weight and ingestion rates
- Assume that receptors obtain all of their food from the site (home range factor of 1.0)
| Table 21F-1. Biota-Sediment Accumulation Factors for Fish and Invertebrates | ||||||
| Analyte | Sediment Invertebrate Bioaccumulation Factors | Fish Bioaccumulation Factors | ||||
| Conservative | Average | Source | Conservative | Average | Source | |
| Metals | ||||||
| Arsenic | 0.69 | 0.143 | (ORNL 1998) | – | – | |
| Copper | 5.25 | 1.556 | (ORNL 1998) | – | – | |
| Lead | 0.607 | 0.071 | (ORNL 1998) | – | – | |
| Mercury | 2.868 | 1.136 | (ORNL 1998) | – | – | |
| Zinc | 7.527 | 1.936 | (ORNL 1998) | – | – | |
| Pesticides/PCBs/Dioxins | ||||||
| 4,4′-DDD | – | – | 0.28 | (USEPA 2004) | ||
| 4,4′-DDE | – | – | 7.7 | (USEPA 2004) | ||
| Total DDTs | – | – | 7.7 | (USEPA 2004) | ||
| Total Chlordanes | – | – | 4.77 | (USEPA 2004) | ||
| Dieldrin | – | – | 1.8 | (USEPA 2004) | ||
| Endrin | – | – | 1.8 | (USEPA 2004) | ||
| Total PCBs | 6.41E+01 | 3.62E+01 | (ORNL 1998) | (USEPA 2004) | (USEPA 2004) | |
| 2,3,7,8-TCDD | – | – | 0.025 | (USEPA 2004) | ||
| Semivolatile Organic Compounds | ||||||
| Acenaphthene | – | – | 0.29 | (USEPA 2004) | ||
| Acenaphthylene | – | – | 0.29 | (USEPA 2004) | ||
| Anthracene | – | – | 0.29 | (USEPA 2004) | ||
| Benzo(a)anthracene | – | – | 0.29 | (USEPA 2004) | ||
| Benzo(a)pyrene | – | – | 0.29 | (USEPA 2004) | ||
| Chrysene | – | – | 0.29 | (USEPA 2004) | ||
| Dibenzo(a,h)anthracene | – | – | 0.29 | (USEPA 2004) | ||
| Fluoranthene | – | – | 0.29 | (USEPA 2004) | ||
| Fluorene | – | – | 0.29 | (USEPA 2004) | ||
| Naphthalene | – | – | 0.29 | (USEPA 2004) | ||
| Phenanthrene | – | – | 0.29 | (USEPA 2004) | ||
| Pyrene | – | – | 0.29 | (USEPA 2004) | ||
| Sum HMW PAHs | – | – | 0.29 | (USEPA 2004) | ||
| Sum LMW PAHs | – | – | 0.29 | (USEPA 2004) | ||
| Total PAHs | – | – | 0.29 | (USEPA 2004) | ||
| – no data (ORNL 1998). (USEPA 2004g). |
||||||
Calculating Biota-Sediment Accumulation Factors
A BSAF for organic chemicals is a unitless ratio of the lipid-normalized wet weight concentration in tissue to the organic carbon-normalized concentration in surface sediment. For inorganic chemicals, the BSAF is a simple unadjusted ratio. BSAFs are transfer coefficients that relate chemical concentrations in biota to chemical concentrations in sediment (USEPA 2004g). In the BERA, site-specific tissue data (either from field-collected organisms or tissue samples from laboratory bioaccumulation tests) can be used to develop site-specific BSAFs. BSAFs are the ratio of chemical concentrations in tissue and chemical concentration in collocated sediment, as follows:

- Where:
- BSAF(metals) = Biota-sediment accumulation factor for metals (unitless)
- Tissue Concentration = Chemical concentration in tissue (mg/kg or µg/kg)
- Sediment Concentration = Chemical concentration in sediment (mg/kg or µg/kg)

- Where:
- BSAF(organics) = Biota-sediment accumulation factor for organics (unitless)
- Tissue Concentration = Chemical concentration in tissue (mg/kg or µg/kg)
- Percent Lipids = Percent lipids in tissue sample (%)
- Sediment Concentration = Chemical concentration in sediment (mg/kg or µg/kg)
- Percent TOC = Percent total organic carbon of the sediment (%)
Normalizing the tissue and sediment concentrations for the organic chemicals to percent lipids and TOC, respectively, is done because organic chemicals have a tendency to bind to lipids and organic carbon.
When field-collected invertebrate tissue concentrations are not available, BSAFs can be calculated using site-specific sediment concentrations and tissue concentrations measured in laboratory bioaccumulation tests. The BSAFs can then be used to estimate the concentrations of chemicals that could occur in invertebrates exposed to average concentrations in sediment in each DU. BSAFs incorporate the percent lipid in tissue and total organic carbon (TOC) in sediment to predict the total concentration in the prey tissue. Although separate site-specific BSAFS for each species of interest would be ideal, application of BSAFs derived for one laboratory invertebrate test species to other invertebrates within the study area is considered appropriate because BSAFs for benthic invertebrates have been shown to be relatively insensitive to interspecific variability (Tracey and Hansen 1996; Burkhard et al. 2010).
Differences in BSAFs in site samples and other sites reported in the literature may be explained by numerous physical, chemical, and biological factors. Bioaccumulation of PCBs and other compounds with high log Kow may differ from what is predicted by equilibrium partitioning because sediment ingestion by the organism may enhance bioavailability in ways not accounted for by log Kow and similar physico-chemical models (Sormunen et al. 2008). Empirical BSAFs derived from site-specific samples are considered a reliable indicator of bioavailability of chemicals in sediment and a direct measure of bioaccumulation under laboratory conditions. The differences between estimated and field-derived BSAFs are lowest between the same species at different locations within a site, or different species at a single site. The extrapolation of BSAFs within or among species at distant unrelated sites decreases the reliability of the estimated BSAF (Burkhard et al. 2010).
Evidence for chemical uptake from sediment includes BSAFs greater than 1.0.
Numerous sources of uncertainty are associated with the derivation, application, and interpretation of benthic invertebrate BSAFs. (Judd et al. 2014) concluded a review of more than 200 BSAFs with words of caution against the over-reliance on BSAFs. In particular, BSAFs should not be extrapolated beyond the chemical concentration on which they were based because the relationship may not be linear. Likewise, the BSAF curve intercept may not be zero. Lastly, one of two outlier concentrations can skew the BSAFs. While an understanding of the influence of lipid concentration on BSAFs may improve the interpretation of bioavailability for some lipophilic compounds, in wild populations lipid concentrations can vary dramatically with season, diet, and reproductive stage (Beckvar and Lotufo 2011). Lipid-adjusted tissue concentrations are not reliably more predictive than standard wet weights for interpreting bioaccumulation processes or toxicity in wild organisms (Wenning et al. 2011). Nevertheless, investigators require some approach to measuring bioaccumulation, and BSAFs can be useful within the limits of these known liabilities (Judd et al. 2014).
A review of publications on bioconcentration factors and bioaccumulation factors for hundreds of organic compounds and test species concluded that field-derived BAFs may be higher than laboratory-derived BAFs (Arnot and Gobas 2006). Conversely, a more directly relevant side-by-side laboratory and in-situ comparison of BSAFs and BAFs using Lumbriculus reported that the two measures were comparable for PCBs (Beckingham and Ghosh 2010).
BSAFs based on field collected data will typically be less accurate that BSAFs derived from laboratory studies because the exposure point concentration for field collected organisms is less certain than it is for laboratory studies. For example, even though non-mobile organisms like mollusks live in the sediment, they are filter feeders and get their exposure from chemicals in the overlying water. While some of the contaminants in the overlying water may be from the adjacent sediment, in aquatic systems, water and sediment are transient so exposure will change over time. Therefore, sediment that is co-located with organisms collected in the field may not accurately represent exposure of the organisms. This is a much greater source of uncertainty with mobile organisms such as crabs and fish.
HDOH recommends that BSAFs should generally only be calculated for detected and non-rejected data. Non-detected data can be used to calculate average sediment concentrations over an exposure area (an MIS approach to sediment sampling reduces the likelihood of obtaining non-detect data), however, non-detected data should not be used to calculate BSAFs if either the tissue or sediment concentrations were non-detect.
