
Image from CDC site
Preventing Prescription Drug Overdose in Hawai`i
What is the Hawai`i Prescription Monitoring Program?
The Hawai`i Prescription Monitoring Program (PMP) is an online system that allows medical providers — both prescribers and pharmacists — to monitor patients’ prescriptions for Schedule II-IV controlled substances, to help prevent prescription drug misuse, and improve quality of care. While PMPs alone are not enough to reduce overdose rates or deaths, they are among the most promising clinical tools available to address prescription drug abuse (DHHS 2013). They have been shown to improve clinical decision-making, reduce doctor shopping and diversion of controlled substances, and help curb the prescription drug abuse epidemic.
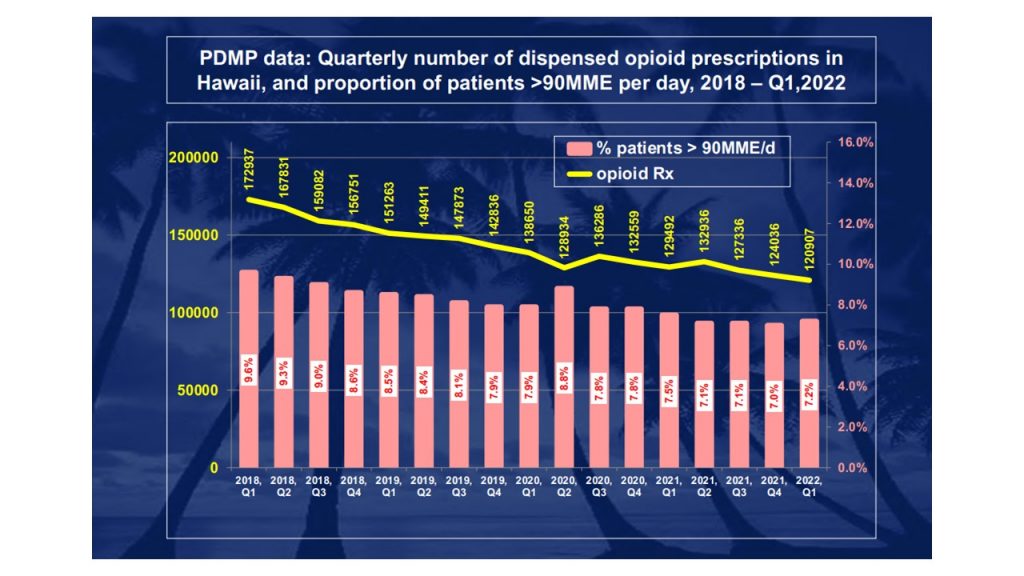
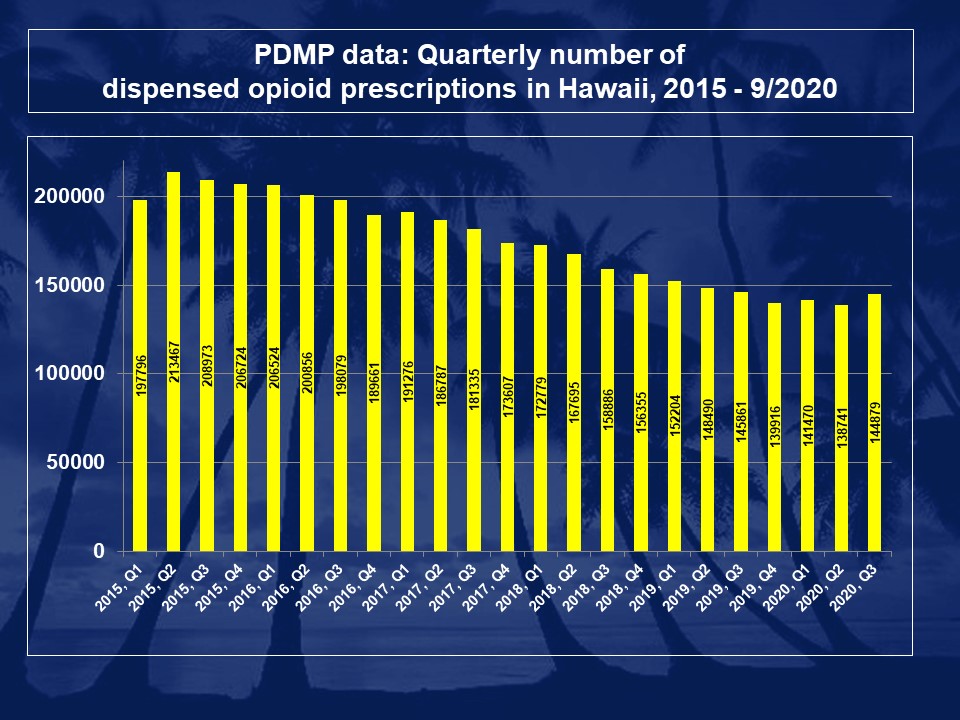
Who is required to register with the Hawaii Prescription Drug Monitoring
Program?
Any registrant of the Narcotics Enforcement Division (NED) that holds a controlled
substance license (except pharmacies and veterinarians) must register as a data
requestor through the AWARxE website. Registrants who dispense controlled
substances will need to register through the Clearinghouse website as a data submitter
(except veterinarians). (HRS Chapter 329-101)
Why Should Prescribers Use the PMP?
- Improve patients’ quality of care, particularly when patients are being treated by more than one provider
- Support access to legitimate medical use of controlled substances
- Prevent drug abuse and diversion
- Identify patients who may be addicted to prescription drugs and help them get treatment
- Increase confidence in prescribing or denying controlled substances
What is the Concern for Hawai`i?
- In recent years, drug poisonings have surpassed motor vehicle crashes as the leading mechanism of injury death in Hawaii.
- Each year in our state, drug poisonings are responsible for an average of 155 deaths and 4,500 hospital treatments.
- Pharmaceutical opiates are involved in at least 41% of fatal drug poisonings and 12%-20% of nonfatal drug poisonings.
More Information About this Public Health Problem and Your Role as a Medical Provider
- CDC’s Injury Center, Prescription Drug Overdose — Surveillance data, guidelines for prescribing opioids, policy impact, state laws, prevention activities, related publications
- Centers of Excellence for Physician Information (NIH) — Course materials on a diverse set of topics, including prescription drug abuse, created through a partnership between the National Institute on Drug Abuse (NIDA) and eight medical universities.
- 2018 and 2019 NEW & CHANGES TO THE LAW
-
Act 95 2019/SB 1263 Amends the Uniform Controlled Substance Act by updating Schedule V and amending requirements for electronic prescriptions, for consistency with federal law.
-
Act 230 2019/SB1486 Amends Electronic Prescription Accountability system (HI-PDMP)
-
Act 251 2019/HB665 Exceptions to query of Electronic Prescription Accountability System (HI-PDMP)
-
Act 256 2019/SB536 Prescription requirements related to schedule II drugs
-
Act 95 2019/SB 1263 Addition of FDA approved CBD drugs to Schedule V
-
Act 066/Senate Bill 505(2017) Effective 07-01-18. Requirement for policy or and written agreements to engage in an informed consent process between the prescribing provider and qualifying opioid therapy patient. Related Resource: Hawaii Department of Health, Patient Informed Consent Form also the Hawaii Revised Statute (HRS) 329.38-5
-
Act 151/House Bill 1602 Effective 08-01-18. Requires the inclusion of a label warning of the risks of addiction and death on the packaging of any opioid drug dispensed by a health care professional or pharmacist.
-
Act 153/Senate Bill 2646 Effective 07-01-18 Pertains to practitioner utilization requirements of the PDMP
-
Act 190/House Bill 2385 Effective 07-01-18. Relating to scheduling actions of substituted cathinones, cumyl-4-cn-binaca and dronabinol
-
- TEMPLATE for Hawaii’s Informed Consent For Opioid Prescribed Pills Form
- Medline Plus (NIH) — Facts, tools, resources, and references that address the problem, diagnosis/symptoms of addiction, treatment, prevention/screening.
- Facing Addiction – The Surgeon Generals Spotlight on Opioids September 2018 The Spotlight on Opioids assembles opioid-related information from the Surgeon General’s Report on Alcohol, Drugs, and Health into one document to better inform the general public, especially family and friends of people with an elevated risk of opioid overdose, opioid misuse, and/or opioid use disorder.
- Opioid Overdose Prevention Toolkit – 2018 (Substance Abuse and Mental Health Services Administration) — Aimed at communities and local governments, this kit includes materials to develop policies and practices to help prevent opioid-related overdoses and deaths. Addresses issues for first responders, treatment providers, and those recovering from opioid overdose.
- TakeAsDirected (Washington State Department of Health) — resources, course materials, assessment tools, and guidelines for healthcare providers and subpopulations

- Summer 2022 Issue
- Fall 2022 Issue
More Information About the Effectiveness of Prescription Drug Monitoring Programs
-
Policy Changes Could Bolster Prescription Drug Monitoring Programs, April 2020 (The PEW Charitable Trusts)
- Prescription Drug Monitoring Programs: Evidence-based practices to optimize prescriber use, December 2016 (The PEW Charitable Trusts)
- Research Update: State prescription drug monitoring programs and drug overdose deaths. October 2017 (CDC’s Injury Center)
For more information on how Hawaii is Preventing Prescription Drug Overdose go to the Strategy, Projects and Community Collaborations section in the Information On Poisoning Prevention tab
Contact:
Tammie.Smith@doh.hawaii.gov
Tammie Healani Hoapili Smith MPH – Data Driven Prevention Initiative Coordinator

