Hawaii Laboratory Response Network
- Established on October 2000 with seven hospital-based and commercial laboratories mostly from Oahu participating.
- Hawaii Laboratory Response Network (HI LRN) includes other hospital-based clinical laboratories in the neighbor Islands, one private food testing laboratory, four veterinary laboratories including the State Veterinary Diagnostic Laboratory of the Department of Agriculture, the Tripler Army Medical Center (TAMC) and the Navy Environmental Preventive Medicine Unit-6 (NEPMU-6).
Goals of the HI LRN
- To build and foster relationships with clinical, military, veterinary, and food testing laboratories.
- To develop a well-trained workforce that is prepared to detect and respond quickly and appropriately to BT and other public health emergencies.
- To maintain surge capacity during a BT incident and other public health emergencies.
- To improve our ability to respond to BT, emerging infectious diseases and other public health emergencies.
- To ensure secure communication and secure electronic exchange of data, information, laboratory report.
The LRN: Laboratory Response Network For Bioterrorism
The Laboratory Response Network (LRN) is a consortium and partnership of laboratories that provide immediate and sustained laboratory testing and communication in support of public health emergencies, particularly in response to acts of bioterrorism. The LRN is currently comprised of local, state, and federal public health laboratories in addition to private and commercial clinical laboratories, and selected food, water, agricultural, military, and veterinary testing laboratories. Other key federal partners include the Federal Bureau of Investigation (FBI), the Department of Defense (DOD), the Environmental Protection Agency (EPA), the Department of Agriculture (USDA), the Department of Justice (DOJ), the Department of Energy (DOE), the Food and Drug Administration (FDA), the Association of Public Health Laboratories (APHL), the National Institutes of Health (NIH), the American Association of Veterinary Laboratory Diagnostics (AAVLD), and the American Society for Microbiology (ASM). All Preliminary testing and screening are performed primarily in a distributed rather than a centralized fashion to ensure a prompt and rapid initial response; a system of triage and referral of specimens ensures transfer of appropriate materials to specialty laboratories where sophisticated equipment, technologies, and expertise are applied to specimen analysis.
The goals of the LRN are to:
- Ensure that the nation’s public health, clinical, and other select laboratories are prepared to detect and respond to a bioterrorism or chemical terrorism event in an appropriate and integrated manner.
- Ensure that all member reference laboratories collectively maintain state-of-the-art biodetection and diagnostic capabilities and surge capacity as well as secure electronic communication of test results for the biological and chemical agents likely to be used in the commission of a biocrime or bioterrorism event.
- Work with other departments and agencies to ensure a successful federal response to an act of bioterrorism and to facilitate and optimize the ability of states to competently respond independently to biocrimes or public health emergencies in the state.
- Promote the CDC’s and DHHS'(Department of Health and Human Services) bioterrorism research agenda and CDC’s internal response needs.
- Enlist and optimal number of registered participating LRN laboratories throughout the U.S. as determined by the LRN working group.
The LRN maintains the following:
- A registry and linkage of clinical and private laboratories in the U.S. that would include Sentinel and Reference laboratories.
- Complete, accurate, and standardized protocols for all levels of testing for agents deemed critical and likely to be used in the commission of biocrimes or acts of bioterrorism.
- Secure but accessible supply of standardized reagents and diagnostic technologies produced and maintained by the CDC.
- Secure electronic laboratory reporting that integrates with key epidemiologic, surveillance, and emergency response components.
- Training and proficiency testing essential to the diagnostic process.
Clinical laboratories play a critical role in the LRN. Their heightened awareness to the possibility of recovering the agents of bioterrorism from patient specimens and referral of suspect isolates to the appropriate public health reference laboratory is crucial (see ASM’s Laboratory Guideline on Packing and Shipping Infectious Substances, Diagnostics Specimens, and Biological Agents, which can be downloaded from the ASM website).
Bioterrorism is defined as the “intentional use of microorganisms, or toxins, derived from living organisms to produce disease and death in humans, animals, or plants.” A bioterrorism event may be either overt or covert.
An overt attack would be accompanied by an announcement that a specific agent was released. These attacks elicit an immediate response by law enforcement and HAZMAT personnel. Public health officials will also be involved to assist in evaluating the risk and control of the disease. Samples (environmental, food, water, animals) for testing would be submitted directly to a public health reference laboratory, usually a state health laboratory.
A covert attack is the release of an organism or toxin without an announcement. Days or weeks may pass before the release is noticed. The event would probably be signaled by a cluster of disease appearing after the incubation period. Emergency departments may be the first to observe unusual patterns of illness while clinical laboratories first cases of disease and raise suspicion of a possible event. Organisms isolated by the clinical laboratory must be forwarded to the appropriate LRN reference laboratory, and public health officials are to be notified of the suspicious event that may be indicative of a bioterrorism incident. Public health officials in concert with law enforcement officials would determine if an attack has occurred, in addition to confirming the identification of the agent, and institute protective and preventive measures designed to minimize the spread of disease.
Classification of LRN Laboratories
The classification of bioterrorism laboratories by the LRN was revised in 2003. The original designations of Level A, B, C, and D laboratories is no longer recognized and are now designated as Sentinel Reference, and National Laboratories (Fig. 1).
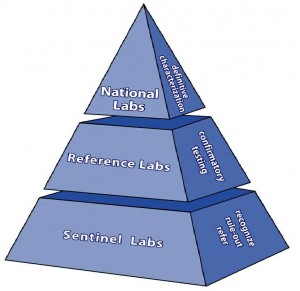
Sentinel (formerly Level A)
Sentinel laboratories are clinical laboratories that follow Biosafety Level 2 (BSL-2) guidelines. Their primary responsibility is to recognize and rule out or refer suspicious agents by following standardized Sentinel Laboratory Guidelines provided on the ASM website. Even though many Sentinel laboratories are capable of providing a “presumptive identification” of some of the targeted organisms, they must refer isolates to an LRN Reference laboratory.
Sentinel Laboratories: Depending on the level of diagnostic testing, there are two kinds of sentinel clinical laboratories. Advanced sentinel clinical laboratories function at the local front line and have the most capability. Laboratories with less analytical capability that are also in a position to handle specimens that might contain agents of bio-terrorism or emerging infectious disease are referred to as basic sentinel clinical laboratories. These latter facilities need at a minimum a communication link to each jurisdiction’s LRN reference laboratory for completion of the network. Characteristics of these two kinds of laboratory are as follows:
Advanced Sentinel Clinical Laboratory
- The laboratory is certified under the Clinical Laboratory Improvement Amendments of 1988 (CLIA) by the Centers for Medicare & Medicaid Services (CMS) for the applicable subspecialty within the specialty of microbiology, and meets the requirements to perform high complexity testing.
- The laboratory is inspected successfully by CMS, a CMS agent, a CMS-approved accreditation organization, or, for a CLIA-exempt laboratory, by that laboratory’s State.
- The laboratory has policies and procedures for direct referral of suspicious specimens or isolates to the nearest LRN reference laboratory in its jurisdiction.
In addition to the above criteria, Advanced Sentinel Laboratories shall meet the following:
- Have a Class II or higher Certified Biological Safety Cabinet.
- Comply with Biosafety Level II (BSL-2) practices1 AND
- Have policies and procedures in place for use of additional respiratory protection, including a definition of when such use is necessary, as well as documentation of safe use (e.g. N-95 fit-testing).
- Have policies and procedures that include the LRN Sentinel Level Clinical Microbiology Laboratory Guidelines that are available and can be downloaded from the ASM website.
- All personnel have been trained, with demonstrated competency, and are fully aware of the details contained within each LRN Sentinel Level Clinical Microbiology Laboratory Guideline.
- Personnel have been trained and certified in Packing and Shipping of Infectious Substances Guidelines2.
- Have procedures to track and account for decontamination of laboratory biological waste (specimens, cultures). At a minimum, ensure that any contract or procedure for waste/disposal is available for inspection in the laboratory safety or waste disposal manual.
It is further highly recommended that Advanced Sentinel Laboratories comply with the following:
- The microbiology laboratory operates under negative pressure as recommended by the American Institute of Architecture (AIA) Guidelines for Construction of Healthcare Facilities3. If a microbiology laboratory is planning to remodel or construct a new facility, it should be designed to operate under negative air pressure as recommended by the AIA. This only applies to new construction or remodeling.
- There is on-site terminal decontamination capability, e.g., autoclaving, for disposal of wastes categorized as BSL-3 or Select Agent.
Basic Sentinel Clinical Laboratory
- The laboratory is certified under the Clinical Laboratory Improvement Amendments of 1988 (CLIA) by the Centers for Medicare & Medicaid Services (CMS) for the applicable subspecialty within the specialty of microbiology.
- The laboratory is inspected successfully by CMS, a CMS agent, a CMS-approved accreditation organization, or, for a CLIA-exempt laboratory, by that laboratory’s State.
- The laboratory has policies and procedures for referral of diagnostic specimens to an Advanced Sentinel Laboratory.
- The laboratory has policies and procedures for direct referral of suspicious specimens or isolates to the nearest LRN reference laboratory in its jurisdiction.
LRN Reference Laboratories
LRN reference laboratories are local and state public health laboratories, selected academic- or university-based laboratories, designated specialty laboratories (veterinary, water, food, chemical, military, agricultural) that possess the reagents and technology for definitive confirmation of organisms including toxin testing, referred by Sentinel laboratories. LRN Reference laboratories follow BSL-3 containment and practice guidelines. Contact your designated state public health laboratory for more information about the LRN reference laboratory located closest to you.
LRN National Laboratories (formerly Level D)
LRN National Laboratories are Federal laboratories that have BSL-4 containment facilities and practice guidelines. The primary laboratory at this level is located at the CDC and specializes in the isolation and identification of BSL-4 agents such as Ebola, Marburg, and Smallpox virus. This laboratory also possesses the capability of advanced genetic characterization and archiving of all bioterrorism agents.
The Clinical Laboratory’s Responsibility
As members of the LRN, Sentinel laboratories have access to the network and serve as “sentinels” for the early detection of and raising suspicion regarding a suspicious agent that cannot be ruled out as a possible bioterrorism-associated organism. Sentinel laboratories do not have access to the CDC secure website for Reference Laboratory Testing Protocols or reagents. Instead, Sentinel laboratories must utilize standardized testing protocols (ASM Sentinel Laboratory Guidelines) that have been designed to utilize conventional tests to facilitate the “rule out” or “referral” of a suspicious isolate to an LRN Reference laboratory.
The Sentinel laboratory is NOT responsible for and SHOULD NOT make the decision that a bioterrorism event has occurred; that responsibility rests with local, state, and federal health and law enforcement officials. A designated individual within your facility (preferably the Infection Control Officer) should be notified of a suspicious agent, who in turns notifies the enforcement or public health officials. The exception is the need to contact the LRN Reference Laboratory for guidance in the disposition of the suspicious agent prior to referral for confirmatory testing.
NOTE: In no case should the Sentinel laboratory accept environmental (powders, letters, packages), animal, food, or water specimens for examination, culture, or transport for bioterrorism-associated agents. Such specimens should be submitted directly to the nearest LRN Reference laboratory.
Laboratory Notification Protocol For Sentinel Laboratories – Chain of Communication
A. If a possible BT agent is grown in a sentinel laboratory or detected by other laboratory means (as outlined in the laboratory protocols included in this document), place phone calls to the responsible physician and the following individuals, noted below, immediately. Contacting these individuals and the procedures required in the laboratory are NOT one-person tasks. Additional assistance from other microbiologists and laboratory support personnel is essential.
OR
B. If a specimen is submitted for detection of a BT agent as the result of a possible BT event, place phone calls to the individuals noted below immediately.
- Your Microbiology Supervisor
- Your Laboratory Manager
- Your Laboratory Director
- Your Hospital Infection Control Officer
- Infectious Disease Physician or ID Consult from your Hospital
- Hawaii Department of Health- Disease Outbreak Control Division: Disease Investigation Branch @ 586-4586
- State Laboratories Division-BT Microbiologist and Coordinator @ 368-3373 (24/7 cell); work- 453-5993; 453-5994; or pager # 691-4014
Handling of Possible BT Agent (for Sentinel Labs)
- A lead technologist/analyst/Microbiologist or Microbiology Supervisor should be appointed and be notified immediately that a suspected BT specimen or agent is in the laboratory. Laboratory workers are to be informed promptly of the name and medical record number of the person(s) with the suspected infection and, if appropriate, to treat other specimens from the patient(s) appropriately. This must be done in a manner that is in compliance with the Health Insurance Portability and Accountability Act (HIPAA).
- All suspected BT specimens are to be processed in the biological safety cabinet located in [fill in institution-specific information; whenever possible, this should be in a biological safety cabinet in a room that is under negative pressure] while wearing appropriate personal protective equipment, such as gown, gloves, and mask.
- Each of the plates, tubes, and blood culture bottles for which this applies must be labeled prominently: “Possible highly infectious agent: [fill in name of agent]”
- All plates that have been streaked for culture or subculture will be sealed with shrink seal or the equivalent and labeled as in step C above.
- Any growth from specimens is to be manipulated in the biological safety cabinet {fill in institution-specific information} while wearing appropriate personal protective equipment, such as gown, gloves, and mask.
- As the culture is being worked up, the technologist(s) working on the culture(s) must be in close touch with the microbiology supervisor and medical director.
- An identification of the organism is NOT the role of the Sentinel microbiology laboratory. An organism that is consistent with, for example, Yersinia pestis, will be forwarded to a LRN Reference or higher laboratory for definitive identification. Do not perform any more manipulation of the cultures than is absolutely essential.
- Sentinel Laboratory Guidelines for the Presumptive Identification of BT Agents, Bacillus anthracis, Brucella spp., Burkholderia spp., Coxiella burnetii, Francisella tularensis, Yersinia pestis can be found in the ASM website or you may refer to the CDC- Bioterrorism Response Guide for Clinical Laboratories distributed to the sentinel laboratories in 2003.
B-SAFE: Bench Cards-Cover
- Bacillus anthracis
- Brucella spp.
- Burkholderia spp.
- Francisella tularensis
- Select Agent Response Algorithm
- Smallpox
- Toxins: Botulinum Toxin, Ricin Toxin, Staph Enterotoxin B
- Yersinia pestis
Resources
- Frequently Asked Questions About the Laboratory Response Network (LRN)
- Sentinel Level Clinical Microbiology Laboratory Guidelines
1 Biosafety in Microbiological and Biomedical Laboratories, 4th Edition, U. S. Department of Health and Human Services.
2 Federal Register, Part IV, Department of Transportation, 49 CFR Part 172, Hazardous Materials: Security Requirements for Offerors and Transporters of Hazardous Materials; Final Rule.
3 American Institute of Architecture, Guidelines for Construction of Healthcare Facilities, 2001 Edition.