Department of Health issues voluntary recall warning for KinderFarms, LLC’s KinderMed Pain & Fever products due to acetaminophen instability
Posted on Nov 17, 2023 in NewsroomHONOLULU – The Hawaiʻi State Department of Health (DOH) Food and Drug Branch (FDB) is alerting residents to a voluntary recall by KinderFarms, LLC for KinderMed Infants’ Pain & Fever and KinderMed Kids’ Pain & Fever OTC medication products because of acetaminophen instability. These products were sold nationwide, including Hawaiʻi, from drug/pharmacy retailers, supermarkets, direct online sales, and online through major e-commerce sites.
Acetaminophen is a common active ingredient in many pain-relieving medicines. As a result of the potential health risk with acetaminophen being outside of specification, the product may cause acute adverse health effects, including abdominal pain, nausea, vomiting, and/or jaundice at higher doses. To date, there have been no reports of serious adverse events associated with this recall.
The impacted products being recalled are all lots of:
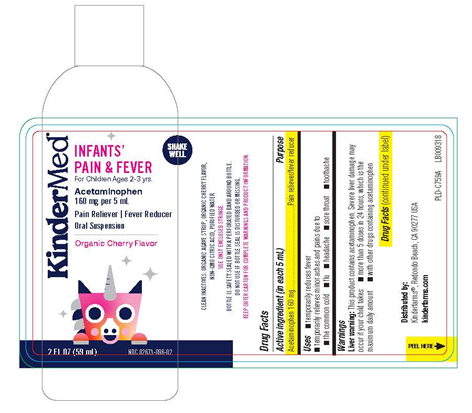
- KinderMed Infants’ Pain & Fever (2 fluid ounces/59 mL), (Acetaminophen – 160 mg per 5 mL), Oral Suspension; UPC: 850001805698
Bottle Label
Box Label

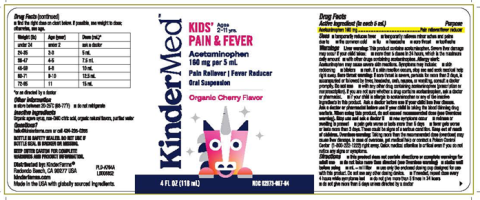
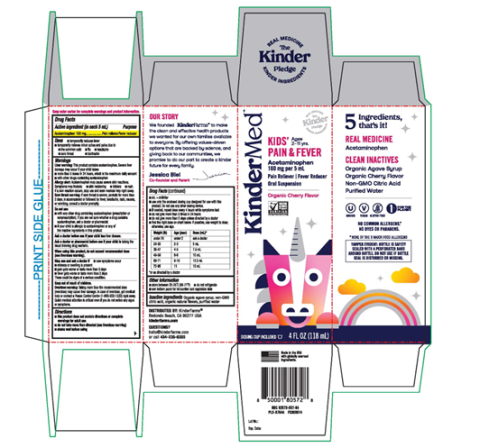
- KinderMed Kids’ Pain & Fever (4 fluid ounces/118 mL), (Acetaminophen – 160 mg per 5 mL), Oral Suspension; UPC: 850001805728
Bottle Label
Box Label
No other KinderFarms, LLC products are impacted by this recall.
The FDB advises consumers to immediately stop using the recalled product. Customers may return the product to the place of purchase for a full refund. For more information, consumers may contact KinderFarms, LLC via email at [email protected] or by calling 1-800-996-2930 from 4:00 AM to 3:00 PM HST.