Statutes and Rules
Contact us:
- Phone: (808) 692-7450
- Email: [email protected]
Hawaii Hemp Task Force
Hawaii Revised Statutes (HRS):
Chapter 328G – Hemp Processors
Hawaii Administrative Interim Rules (HAR):
Please click thumbnails to view informational briefing
- Chapter 11-37 Interim Rules, effective December 6, 2024
- Chapter 11-37 Interim Rules (Ramseyer format), effective December 6, 2024
Hemp Permit Application
Click on this link to download the Hemp Permit Application
Chapter 11-37 summary/announcements:
Updated interim rules, effective December 6, 2024:
Please click thumbnails to view informational briefing
- Summary of Amendments Ch. 11-37 (Interim Rules) Hawaii Administrative Rules – “Hemp Processing and Manufactured Hemp Products”
- Information to Obtain Hemp Processor Permit
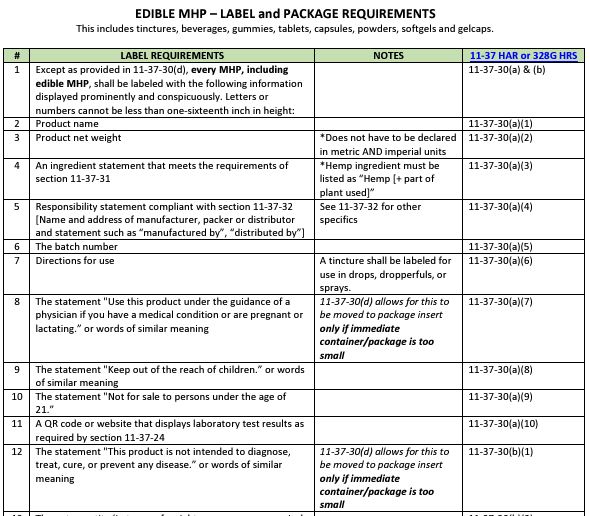
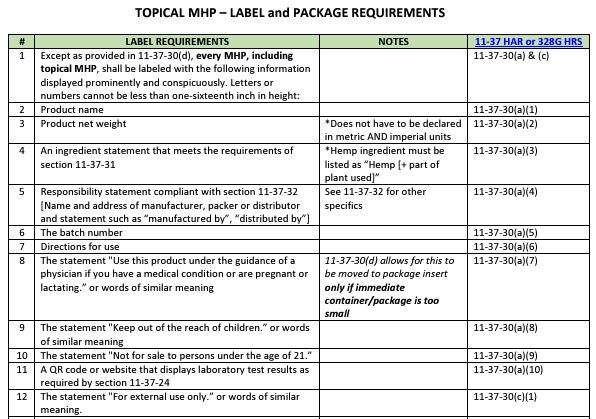
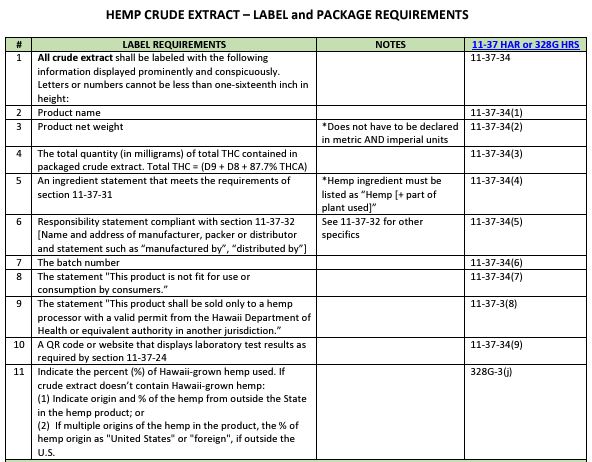
- Label and Package Requirement Guide for permitted hemp processors, retailers, and distributors.
- February 1, 2024, the Committee on Commerce and Consumer Protection, Committee of Health and Human Services, and Committee on Agriculture and Environment held an informational briefing to hear from the Department of Health, Office of Medical Cannabis Control and Regulation on the implementation of Act 263 (2023), including the status of its administrative rules making process. The committee will also hear updates from the Department of the Attorney General, the Cannabis Regulators Association, and the Oregon Liquor and Cannabis Commission on national trends in regulating hemp and cannabinoids.
Updated interim rules, effective April 29, 2022:
- Change definition of total THC to: “Total tetrahydrocannabinol” or “total THC” means the sum of the percentage by weight of:
- (1) Delta-9-tetrahydrocannabinolic acid (D9-THCA) multiplied by 0.877; and
- (2) Delta-9-tetrahydrocannabinol (D9-THC). [§2]
- Changes to laboratory analysis requirements [§23(b)] A sample and the associated batch of hemp product is considered adulterated if laboratory results for the testing required in subsection (a) exceed the specified concentration limit for any of the following contaminants:
- (3) Solvents listed in Table 3, provided that:
(A) The limit for ethanol does not apply to tinctures; and
(B) The limit for ethanol does not apply to hemp products intended for topical application to the skin or hair;
| Solvent | Chemical Abstracts Service Registry Number
(CAS No.) |
Limit (parts per million) |
| Benzene | 71-43-2 | 1.0 ppm |
| Butane | 106-97-8 | 5000 ppm |
| Ethanol | 64-17-5 | 5000 ppm |
| Heptane | 142-82-5 | 5000 ppm |
| Hexane | 110-54-3 | 290 ppm |
| Pentane | 109-66-0 | 5000 ppm |
| Toluene | 108-88-3 | 890 ppm |
| Total xylenes (ortho-, meta-, para-) | 1330-20-7 | 2170 ppm |
-
- (4) The following microbial contaminants, which must not be detected in one gram of hemp product:
- removed Aspergillus terreus
- removed Aspergillus terreus
- (4) The following microbial contaminants, which must not be detected in one gram of hemp product:
- Conforming change removing reference to limit for isopropyl alcohol [§23(b)(3)(B)]
Updated interim rules, effective February 24, 2022:
2. Align with medical cannabis program requirements
- Contaminant testing
- Ingredient prohibition: cannabinoids created through isomerization, including delta-8-tetrahydrocannabinol and delta-10-tetrahydrocannabinol
- Testing and labeling for total THC
3. Remove testing and labeling requirements for THCA and CBDA
- Still require testing and quantity labeling for “Any other cannabinoid specifically listed, described, or advertised in the label or packaging”
Effective August 9, 2021:
These rules are the next step toward regulating the growing hemp industry in Hawaii in a way that provides local hemp farmers a legal pathway to bring consumable hemp products to market while protecting consumers by requiring lab testing for contaminants and labeled cannabinoid content.
While not a complete summary of the rules, the information below is intended to assist those planning to process hemp into hemp products in Hawaii as well as those planning to sell finished hemp products in Hawaii.
- Prior to processing hemp into hemp products, USDA licensed hemp producers must apply to be on the hemp processor registry, administered by the DOH Food and Drug Branch (FDB), and receive a certificate of registration.
- Registered hemp processors must comply with processing practices and facility standards, quality control, record keeping, recall plan, finished product testing, and labeling rules.
- Only hemp products that pass required testing, conducted by a qualified lab, can be sold. Test results must be accessible to consumers via QR code or website address printed on label or packaging.
- Only the sale of properly labeled and tested hemp products containing naturally occurring cannabinoids, like cannabidiol (CBD), are allowed when intended:
- To be consumed orally to supplement the diet in tablet, capsule, powder, softgel, gelcap or liquid form (e.g. hemp oil); or
- For topical application to skin or hair.
- In addition to product name, ingredient, and cannabinoid content requirements, product labeling must include advisory/warning statements.
- Sale of the following is prohibited:
- Hemp products with delta-9 tetrahydrocannabinol (THC) above 0.3%.
- Hemp-containing products intended to be consumed orally in a form other than tablet, capsule, powder, softgel, gelcap or liquid form (e.g. hemp oil). Gummies are not allowed.
- Foods and beverages (including bottled water) containing hemp derivatives like CBD and other cannabinoids.
- Cannabinoid-containing products intended to be aerosolized and inhaled (i.e. vape liquids containing cannabinoids).
- Hemp flower material, hemp leaf, hemp cigarettes, etc. intended to be smoked or inhaled.
- Hemp-containing products that are intended to be introduced into the body via eyes, ears, nasal cavities, and other non-oral routes of entry.
DOH strongly encourages all retailers that plan to sell hemp products to work with hemp processors, distributors, and suppliers to ensure finished products follow the product testing and labeling rules found in subchapters 2 and 3 of HAR chapter 11-37. Retailers found selling hemp products out of compliance may be subject to penalties up to $10,000 for each offense, including product removal from sale.